New Geochemical and Palaeontological Data from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sementes Do Gênero Samaropsis Goeppert No Permiano Inferior Da Bacia Do Paraná, Sul Do Brasil
Rev. bras. paleontol. 10(2):95-106, Maio/Agosto 2007 © 2007 by the Sociedade Brasileira de Paleontologia SEMENTES DO GÊNERO SAMAROPSIS GOEPPERT NO PERMIANO INFERIOR DA BACIA DO PARANÁ, SUL DO BRASIL JULIANE MARQUES DE SOUZA & ROBERTO IANNUZZI Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, UFRGS, Cx. P. 15001, 91501-970, Porto Alegre, RS, Brasil. [email protected], [email protected] RESUMO – O afloramento Morro do Papaléo, localizado no município de Mariana Pimentel, Rio Grande do Sul, tem se mostrado uma importante fonte de sementes fósseis preservadas na forma de impressões, as quais são provenientes do Grupo Itararé e da Formação Rio Bonito, Permiano Inferior da bacia do Paraná. Neste estudo são descritos seis diferentes morfotipos para o gênero Samaropsis Goeppert que, possivelmente, correspondem a seis diferentes morfoespécies. Características como a largura da sarcotesta, a forma geral da semente e do nucelo e a textura de sua superfície permitiram a proposição de uma nova espécie, Samaropsis gigas nov. sp., e identificar a presença de Samaropsis kurtzii Leguizamón, S. aff. S. millaniana Oliveira & Pontes e S. aff. S. rigbyi Millan. Além disso, uns poucos espécimes foram classificados apenas a nível genérico, tendo sido designados como Samaropsis sp. 1 e Samaropsis sp. 2. Palavras-chave: Sementes fósseis, Grupo Itararé, Formação Rio Bonito, bacia do Paraná, Rio Grande do Sul. ABSTRACT – SEEDS OF THE GENUS SAMAROPSIS GOEPPERT IN THE LOWER PERMIAN OF THE PARANÁ BASIN, SOUTHERN BRAZIL. The Morro do Papaléo outcrop is located in Mariana Pimentel town, Rio Grande do Sul, and has been an important source of fossil seeds. -

Permophiles Issue #28 1996 1
Permophiles Issue #28 1996 1. SECRETARY’S NOTE I should like to thank all those who contributed to this issue of I have discussed the matter of financial support for Permophiles @Permophiles@. The next issue will be in November 1996 and with many of the members of the Permian Subcommission; the will be prepared by the new secretary, CIaude Spinosa. Please consensus is that we should request voluntary donations to offset send your contributions to him (see note below from Forthcoming processing, mailing and paper costs. We are asking for contribu- Secretary). tions of $10 to $25. However, Permophiles will be mailed to ev- I should like to express my gratitude to those of you who have eryone on the mailing list regardless of whether a contribution is submitted contributions during my eight year term of office. You made. have helped make =Permophiles@ a useful, informative, and timely We have established access to Permophiles via the Internet; the Newsletter of the Subcommission on Permian Stratigraphy. address will be: J. Utting http://earth.idbsu.edu/permian/permophiles Geological Survey of Canada (Calgary) 3303 - 33rd Street N.W. Our intention is to provide a version of Permophiles that is readily Calgary, Alberta, Canada T2L 2A7 available through the Internet. The Internet version of Permophiles Phone (404) 292-7093 FAX (403) 292-6014 will have multiple formats. Initially the format of the Internet ver- E-mail INTERNET address: [email protected] sion will be different from the official paper version. Because new taxonomic names can not be published in Permophiles, a hard copy, downloaded from the interned will suffice for many of us. -

Bioestratigrafía Del Paleozoico Superior De América Del Sur: Primera Etapa De Trabajo Hacia Una Nueva Propuesta Cronoestratigr
Asociación Geológica Argentina Serie D: Publicación especial Nº 11 Bioestratigrafía del Paleozoico Superior de América del Sur: Primera Etapa de Trabajo Hacia una Nueva Propuesta Cronoestratigráfica La impresión de esta publicación fue posible por la colaboración de: la Agencia Nacional de Promoción Científica y Tecnológica a través del PICT-R 00313/1-2 Foto de tapa: Hugo Carrizo. Extremo Norte de la Sierra de Narváez (Sistema de Famatina). Vista hacia el norte del río Chaschuil. Formación De La Cuesta (Pérmico).Sección Superior. Sedimentitas fluviales con estratificación tangencial. Diseño y composición: M. Laura Iribas. Correo: [email protected] Se terminó de imprimir en el mes de septiembre de 2007 en los talleres del Instituto Salesiano de Artes Gráficas, Don Bosco 4053, Buenos Aires, Argentina. ISSN 0328-2767. Reservados todos los derechos. BIOESTRATIGRAFÍA DEL PALEOZOICO SUPERIOR DE AMÉRICA DEL SUR: PRIMERA ETAPA DE TRABAJO HACIA UNA NUEVA PROPUESTA CRONOESTRATIGRÁFICA Convocada por: Paleontólogos de la UBA y de la Fundación Lillo. Auspiciada por: Comité Organizador de la XI Reunião de PaleobotânicosPaleobotânicos y por el Comité Organizador del XIII Simposio Argentino de Paleobotánica y Palinología (Bahía Blanca) Argentina 2006. Comité organizador local Dr. Carlos L. Azcuy CONTENIDO Prólogo .......................................................................................................................................................................................... 5 Introducción ............................................................................................................................................................................. -

Pb-651 PC Srivastava1
The Palaeobotanist 57(2008) : 119-139 0031-0174/2008 $2.00 Some aspects of Palaeozoic pteridophytes of India: A critical reappraisal PRADEEP CHANDRA SRIVASTAVA Department of Botany, Ewing Christian College, University of Allahabad, Allahabad 211 003, India. Email: [email protected] (Received 03 August, 2006; revised version accepted 07 December, 2007) ABSTRACT Srivastava PC 2008. Some aspects of Palaeozoic pteridophytes of India: A critical reappraisal. The Palaeobotanist 57(1-2) : 119-139. The present paper deals with spatial and temporal distribution of pteridophytic megafossils within Palaeozoic plant bearing horizons of India. An attempt has been made to describe the pteridophytic assemblages in various strata of different ages, dynamism of the relative dominance pattern of various types of pteridophytes within each assemblage vis- à-vis the environment they lived in. Critical discussion has been done regarding increasing diversity of Palaeozoic pteridophytic taxa, taxonomic riddles, plant architecture, ecological analysis and phylogenetic implications. Special attention has been given to the floristic regionalism of Lower Carboniferous flora of India in reference to the world palaeophytogeography and occurrence of admixture of Cathaysian, Euramerian and Gondwana pteridophytic elements in palaeoecotone zone near the northwestern boundary of Indian Gondwana Plate. Key-words—Lycopods, Sphenopsids, Pteridophylls, Extra-Peninsular and Peninsular, Pre-Gondwana, Lower Gondwana. Hkkjr ds iqjkthoh VsfjMksQk;Vksa ds dqN igyw -

Three New Fern Fronds from the Glossopteris Flora of India
THREE NEW FERN FRONDS FROM THE GLOSSOPTERIS FLORA OF INDIA P. K. MAITHY Birbal Sahni Institute of Palaeobotany, Lucknow-226007 ABSTRACT of dates, Damudopteris becomes synonym A new species of Neomariopteris and a new to Neomariopteris, because the former form species of Dichotomopteris is recorded. In addition has been published one month later. to this a new genus Santhalea is instituted. INTRODUCTION Neomariopteris khanU sp. novo Diagnosis - Fronds large, at least tri• the ferns from the Lower Gondwanas knowledge on the morphology of pinnate; catadromic, rachis winged, secon• OURof India has been advanced consider• dary rachis broad, emerge alternately at an ably from the recent work of Maithy (1974a, angle of ± 60°; pinnae lanceolate; attached 1974b, 1975), Pant and Misra (1976) and alternate, sub-opposite or opposite from Pant and Khare (1974). Recently Maithy secondary rachis; lateral pinnules ovate, has revised the Lower Gondwana ferns from 1'0 cm long and 0'4 mm broad at base, i.e. India. On the basis of his revision, he has the length and breadth ratio of the pinnules instituted two new genera Neomariopteris is 2·5: I, lateral pinnules alternately and Dichotomopteris and an unrecorded form arranged, standing at right angles to rachis, Dizeugotheca. Pant and Khare (1974) and decurrent, attached by broad bases, lateral Pant and Misra (1976) have reported two fusion of two pinnules margin is ± 1/4 length new genera Damudopteris and Asansolia from of the pinnules from the base; apex acute; the Raniganj Coalfield. margin entire; both the margins show out• The present paper deals with three new ward curvature; terminal pinnules smaller fern fronds collected recently from than lateral pinnules, triangular in shape. -

Bioestratigrafía Del Pérmico De La Sierra De Los Llanos, La Rioja (Cuenca Paganzo), Sobre La Base De La Megaflora Y Su Correlación Con Áreas Relacionadas
Bioestratigrafía del Pérmico de la Sierra de Los Llanos, La Rioja (Cuenca Paganzo), sobre la base de la megaflora y su correlación con áreas relacionadas Eliana Paula Coturel Tesis para optar por el título de Doctor en Ciencias Naturales Director : Dr. Pedro Raúl Gutiérrez Codirector : Dr. Eduardo Morel Facultad de Ciencias Naturales y Museo Universidad Nacional de La Plata - 2013 - ELIANA PAULA COTUREL – BIOESTRATIGRAFÍA DEL PÉRMICO DE LA SIERRA DE LOS LLANOS … Quiero dedicar esta Tesis a mi mamá, Mónica Edith Emmerich. Creo que no hace falta dar razones de por qué una le dedica su Tesis a su Madre. Tampoco hay, en el rico idioma castellano, palabras suficientes para hacerlo. Agradezco a todos los que hicieron posible el desarrollo de esta Tesis. A mis directores, los Dres. Pedro R. Gutiérrez y Eduardo M. Morel, por la confianza depositada y por permitirme trabajar y aprender bajo su ala. A mis compañeras de equipo, Lucía Balarino y Bárbara Cariglino, por la ayuda y el apoyo que me dieron en todos estos años (nota aquí: Bárbara escaneó las láminas de Glossopteris que usé en el capítulo de morfografía, es importante mencionarlo). Las quiero mucho. Y a Gustavo Correa, por el apoyo en el viaje de campo y el tiempo que compartimos en el Museo. Lo normal sería ver aquí un listado de gente y miles de agradecimientos personales, pero a mí no me parece que sea el lugar (principalmente porque seguro que me olvido de alguien y queda muy feo al estar escrito!!). A cada persona que quiero, que aprecio, se lo hago saber (quizás no con palabras, pero sí con los gestos). -
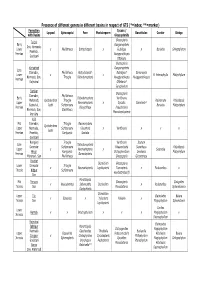
Presence of Different Genera in Different Basins in Respect of GTS
Presence of different genera in different basins in respect of GTS (*=index; **=marker) Formation Cycads / Lyopod Sphenopsid Fern Pteridosperm Benettiales Conifer Ginkgo with Basins Glossopterids Glossopteris Talchir Early Gangamopteris Son, Narmada, Lower x Phyllotheca Botrychiopsis x Rubidgea x Buriadia Ginkgophyton Pranhita, Permian Noeggerothiopis Godabari Ottokaria Glossopteris Karharbari Gangamopteris Late Damodar, Phyllotheca Botrychiopsis* Rubidgea* Samaropsis Lower x x B. heterophylla Platyphyllum Narmada, Son, Trizygia Dichotomopteris Noeggerothiopis Noeggarothiopsis Permian Rajmahal Ottokaria* Euryphyllum Barakar Glossopteris Damodar, Phyllotheca Early Dichotomopteris Vertibraria Mahanadi, Cyclodendron Trizygia Walkomella Rhipidopsis Upper Neomariopteris x Cycads: Barakaria* Rajmahal, lesbii Schizoneura Buriadia Platyphyllum Permian Dizagotheca Pseudocteris Narmada, Son, Stellotheca Macrotaeniopteris Pranhita Kulti Mid Damodar, Trizygia Neomaripteris Cyclodendron Upper Narmada, Schizoneura Gleopteris x Vertibraria x x x lesbii Permian Pranhita, Raniganjia Santalia Godabari Raniganj Trizygia Vertibraria Scutum Late Dichotomopteris Damodar Schizoneura Palaeovittaria Senotheca Rhipidopsis Upper x Neomariopteris x Searsolia Hingir Raniganjia Dictyopteridium Denkania Platyphyllum Permain Damodopteris Mahanadi, Son Phyllotheca Glossopteris Glossotheca Panchet Dicroidium Glossopteris Lower Damodar Trizygia x Neomariopteris Lepidopteris Taenopteris x Podozanites x Triassic Nidpur Schizoneura Kendostrobus(f) Son Marattiopsis Mid Parsora -

Challenges in Indian Palaeobiology
Challenges in Indian Palaeobiology Current Status, Recent Developments and Future Directions © BIRBAL SAHNI INSTITUTE OF PALAEOBOTANY, LUCKNOW 226 007, (U.P.), INDIA Published by The Director Birbal Sahni Institute of Palaeobotany Lucknow 226 007 INDIA Phone : +91-522-2740008/2740011/ 2740399/2740413 Fax : +91-522-2740098/2740485 E-mail : [email protected] [email protected] Website : http://www.bsip.res.in ISBN No : 81-86382-03-8 Proof Reader : R.L. Mehra Typeset : Syed Rashid Ali & Madhavendra Singh Produced by : Publication Unit Printed at : Dream Sketch, 29 Brahma Nagar, Sitapur Road, Lucknow November 2005 Patrons Prof. V. S. Ramamurthy Secretary, Department of Science & Technology, Govt. of India Dr. Harsh K. Gupta Formerly Secretary, Department of Ocean Development, Govt. of India Prof. J. S. Singh Chairman, Governing Body, BSIP Prof. G. K. Srivastava Chairman, Research Advisory Committee, BSIP National Steering Committee Dr. N. C. Mehrotra, Director, BSIP - Chairman Prof. R.P. Singh, Vice Chancellor, Lucknow University - Member Prof. Ashok Sahni, Geology Department, Panjab University - Member Prof. M.P. Singh, Geology Department, Lucknow University - Member Dr. M. Sanjappa, Director, Botanical Survey of India - Member Dr. D. K. Pandey, Director (Exlporation), ONGC, New Delhi - Member Dr. P. Pushpangadan, Director, NBRI - Member Prof. S.K. Tandon, Geology Department, Delhi University - Member Dr. Arun Nigvekar, Former Chairman, UGC - Member Dr. K.P.N. Pandiyan, Joint Secretary & Financial Adv., DST - Member Local Organizing Committee Dr. N. C. Mehrotra, Director - Chairman Dr. Jayasri Banerji, Scientist ‘F’ - Convener Dr. A. K. Srivastava, Scientist ‘F’ - Member Dr. Ramesh K. Saxena, Scientist ‘F’ - Member Dr. Archana Tripathi, Scientist ‘F’ - Member Dr. -

The Plant Ofneomariopteris Hughesii (Zeille'r) Maithy
P"/O(,O/JO/(IJ/;S/ -18 ([ 999 ) 225-238 Oil.'! -Il 17.J1'J9i225-DS $200 The plant ofNeomariopteris hughesii (Zeille'r) Maithy KAMAL JEET SINGH AND SHAlLA CHANDRA 8i,-!Jo/ Sollni InsfiflllC ofPo/nco!Jo/(/n)'. 53 Unil'c,-sity Rood, LucknOlI' 226007, India. (Received 19 M;lrch 1996; revised vCl"sion Jccepted 25 August 1999) ABSTRACT Singh KJ & Ch:mdra S. 1999. The plan! of Neolllilriop/eris hughesii (Zeiller) Maithy. PJlaeobotanist 48(3) : 225-238 An attempt has been m;lde to reconstruct the plant of Neollli/,-iop/eris Ill/ghcsii b;lsed on fifty five hand specimcns collccled frolllihe Barakar Formation exposed ne;lr 13 I'ij raj Nag;lr Railway Station in the Ib-River Coalfield. Orissa. The limit;ltions of this reconstruction have been reJlised by the Juthors as the main trunk of the plont and the fertile structures ;I['e not recorded from this vel'y location, however, combined evidences pUI together from OIher sourceS;lS well suggest that Ihis fern plant could be a slllalltree based on branched stems of consider;lble length ond width ['other thJn a usual prostr;lte rern habit. An up to dale list of allthe specimens recorded under the genus Neo!llilriopleris and its six species by various workers from differenl localities and form;ltions or Jndia has also been given. Key-words-Neo/lwriopleris IliIghesii, Reconstruction, Pinnae, Pinnule, Rachis, Jndio. mn~ ~ ~m ~l3iNn4f~n& ~~ (~) CfiT ~ ~ffi' ."n .... ~"\ .. fB'Q i:RIT ~ ~ ~ q, ~ ~ ~ ~ ~ ~ ;pn: twr R~R ~ R<R JRl'lful ~ B 55 ~ mc:~n ~q, ~ ~ ~ ~ q, JTItn\ q-{ f.rJilijfi47c2f(I'I cftq q\'t m 'fiT t:;'li M Tf1!T I ~ ~ ~ ~ ~ W~ ~ ~ I:ift~ ~ \W( 'liT J:rcIT1<TI 'fiT BfIflT 'T9T cftq 'fiT Cfqr \3CIT ~ ~ ~ ~ ~ B c& "ll 00, \3Pi Will B m-e<l IfITIUil c€T m B lffilTfcm Ncn %f<l; m 'fiT ~, ~ ~ ~~ ~ ~ ~ ~ cfttlT J:w:Rl ~ CJ:~f m un Cfqr cRT q-{ €PIT Cfqr m-Rc ~ ~ ~ 'fiT m ~ m €PIT I 'l1ml <tr ~ I:ift~ ~ ~ ~ B Fcn~ ffi1if &m 6 ~ ~~n" ~ ~ ~ f.rJilijf(4lt?f(A q'~T q, \Jm q\'t '4l <fT %I INTRODUCTION ern & northern forms hence placed under new generic and specific names. -

Annual Review of Pteridological Research - 2001
Annual Review of Pteridological Research - 2001 Annual Review of Pteridological Research - 2001 Literature Citations All Citations 1. Abramova, L. M. & U. B. Yunusbaev. 2001. Experience in studying synanthropization in the course of pasture disgression in the transural steppes by the transect method. Ekologiya (Moscow) 6: 474-477. [Russian& Equisetum arvense] 2. Acock, P. J., F. J. Rumsey, R. Murphy & I. Bennallick. 2001. Polystichum Xlesliei (P. munitum X setiferum) (Dryopteridaceae: Pteridophyta) described and a second site reported. Fern Gazette 16: 245-251. http://www.nhm.ac.uk/hosted_sites/bps/gazette.htm. 3. Agarwal, N. K. & A. Borah. 2001. On the biodiversity of Bhairab hills of Bongaigaon district of Assam: Part I. Flora. Journal of Economic & Taxonomic Botany 25(2): 247-252. 4. Aguiar, S., J. Amigo, S. Pajaron, E. Pangua, L. G. Quintanilla & C. Ramirez. 2001. Identification and distribution of the endangered fern Blechnum corralense Espinosa. P. 16. In Fern flora Worldwide - threats and responses, an international symposium 23-26 July. University of Surrey, Guildford, UK. [Abstract] 5. Aguraiuja, R. 2001. Study of protected ferns of Estonia in Tallinn Botanic Garden. Studies of the Tallinn Botanic Garden V. Plant and Environment: 85-98. [Estonian] 6. Aguraiuja, R. 2001. Complex study of protected ferns of Estonia to defend natural populations. P. 16. In Fern flora Worldwide - threats and responses, an international symposium 23-26 July. University of Surrey, Guildford, UK. [Abstract] 7. Aguraiuja, R. & M. Liik. 2001. Tallin Botanic Garden in the monitoring program of protected plant species in Estonia (1994- 2000). Studies of the Tallin Botanic Garden V. Plant and Environment. -

The First Record of the Permian Glossopteris Flora from Sri Lanka
Geol. Mag. 155 (4), 2018, pp. 907–920. c Cambridge University Press 2016. This is an Open Access article, distributed 907 under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited. doi:10.1017/S0016756816001114 The first record of the Permian Glossopteris flora from Sri Lanka: implications for hydrocarbon source rocks in the Mannar Basin ∗ ∗ G. EDIRISOORIYA , H.A. DHARMAGUNAWARDHANE & STEPHEN MCLOUGHLIN† ‡ ∗ Department of Geology, University of Peradeniya, Peradeniya, Sri Lanka †Department of Palaeobiology, Swedish Museum of Natural History, Box 50007, S-104 05, Stockholm, Sweden (Received 8 July 2016; accepted 9 November 2016; first published online 13 December 2016) Abstract – Strata exposed near Tabbowa Tank, Tabbowa Basin, western Sri Lanka have yielded the first representatives of the distinctive Permian Glossopteris flora from that country. The assemblage includes gymnosperm foliage attributable to Glossopteris raniganjensis, roots referable to Ve r t e b - raria australis, seeds assigned to Samaropsis sp., sphenophyte axes (Paracalamites australis)and foliage (Sphenophyllum emarginatum), and fern foliage (Dichotomopteris lindleyi). This small mac- roflora is interpreted to be of probable Lopingian (late Permian) age based on comparisons with the fossil floras of Peninsula India. Several Glossopteris leaves in the assemblage bear evidence of ter- restrial arthropod interactions including hole feeding, margin feeding, possible lamina skeletonization, piercing-and-sucking damage and oviposition scarring. The newly identified onshore Permian strata necessitate re-evaluation of current models explaining the evolution of the adjacent offshore Man- nar Basin. -

Cryptic Diversity of a Glossopteris Forest: the Permian Prince Charles Mountains Floras, Antarctica
CRYPTIC DIVERSITY OF A GLOSSOPTERIS FOREST: THE PERMIAN PRINCE CHARLES MOUNTAINS FLORAS, ANTARCTICA by Ben James Slater A thesis submitted to the University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Geography, Earth and Environmental Sciences College of Life and Environmental Sciences University of Birmingham September 2013 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT The Toploje Member chert is a Roadian to Wordian autochthonous– parautochthonous silicified peat preserved within the Lambert Graben, East Antarctica. It preserves a remarkable sample of terrestrial life from high-latitude central Gondwana prior to the Capitanian mass extinction event from both mega- and microfossil evidence that includes cryptic components rarely seen in other fossil assemblages. The peat layer is dominated by glossopterid and cordaitalean gymnosperms and contains sparse herbaceous lycophytes, together with a broad array of dispersed organs of ferns and other gymnosperms. The peat also hosts a wide range of fungal morphotypes, Peronosporomycetes, rare arthropod remains and a diverse coprolite assemblage. The fungal and invertebrate-plant interactions associated with various organs of the Glossopteris plant reveal the cryptic presence of a ‘component community’ of invertebrate herbivores and fungal saprotrophs centred around the Glossopteris organism, and demonstrate that a multitude of ecological interactions were well developed by the Middle Permian in high-latitude forest mires.