A Study of Chemistry, Dynamics and Dust in Nova Outflows
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JOHN R. THORSTENSEN Address
CURRICULUM VITAE: JOHN R. THORSTENSEN Address: Department of Physics and Astronomy Dartmouth College 6127 Wilder Laboratory Hanover, NH 03755-3528; (603)-646-2869 [email protected] Undergraduate Studies: Haverford College, B. A. 1974 Astronomy and Physics double major, High Honors in both. Graduate Studies: Ph. D., 1980, University of California, Berkeley Astronomy Department Dissertation : \Optical Studies of Faint Blue X-ray Stars" Graduate Advisor: Professor C. Stuart Bowyer Employment History: Department of Physics and Astronomy, Dartmouth College: { Professor, July 1991 { present { Associate Professor, July 1986 { July 1991 { Assistant Professor, September 1980 { June 1986 Research Assistant, Space Sciences Lab., U.C. Berkeley, 1975 { 1980. Summer Student, National Radio Astronomy Observatory, 1974. Summer Student, Bartol Research Foundation, 1973. Consultant, IBM Corporation, 1973. (STARMAP program). Honors and Awards: Phi Beta Kappa, 1974. National Science Foundation Graduate Fellow, 1974 { 1977. Dorothea Klumpke Roberts Award of the Berkeley Astronomy Dept., 1978. Professional Societies: American Astronomical Society Astronomical Society of the Pacific International Astronomical Union Lifetime Publication List * \Can Collapsed Stars Close the Universe?" Thorstensen, J. R., and Partridge, R. B. 1975, Ap. J., 200, 527. \Optical Identification of Nova Scuti 1975." Raff, M. I., and Thorstensen, J. 1975, P. A. S. P., 87, 593. \Photometry of Slow X-ray Pulsars II: The 13.9 Minute Period of X Persei." Margon, B., Thorstensen, J., Bowyer, S., Mason, K. O., White, N. E., Sanford, P. W., Parkes, G., Stone, R. P. S., and Bailey, J. 1977, Ap. J., 218, 504. \A Spectrophotometric Survey of the A 0535+26 Field." Margon, B., Thorstensen, J., Nelson, J., Chanan, G., and Bowyer, S. -

Astronomy Binder
Astronomy Binder Bloomington High School South 2011 Contents 1 Astronomical Distances 2 1.1 Geometric Methods . 2 1.2 Spectroscopic Methods . 4 1.3 Standard Candle Methods . 4 1.4 Cosmological Redshift . 5 1.5 Distances to Galaxies . 5 2 Age and Size 6 2.1 Measuring Age . 6 2.2 Measuring Size . 7 3 Variable Stars 7 3.1 Pulsating Variable Stars . 7 3.1.1 Cepheid Variables . 7 3.1.2 RR Lyrae Variables . 8 3.1.3 RV Tauri Variables . 8 3.1.4 Long Period/Semiregular Variables . 8 3.2 Binary Variables . 8 3.3 Cataclysmic Variables . 11 3.3.1 Classical Nova . 11 3.3.2 Recurrent Novae . 11 3.3.3 Dwarf Novae (U Geminorum) . 11 3.3.4 X-Ray Binary . 11 3.3.5 Polar (AM Herculis) star . 12 3.3.6 Intermediate Polar (DQ Herculis) star . 12 3.3.7 Super Soft Source (SSS) . 12 3.3.8 VY Sculptoris stars . 12 3.3.9 AM Canum Venaticorum stars . 12 3.3.10 SW Sextantis stars . 13 3.3.11 Symbiotic Stars . 13 3.3.12 Pulsating White Dwarfs . 13 4 Galaxy Classification 14 4.1 Elliptical Galaxies . 14 4.2 Spirals . 15 4.3 Classification . 16 4.4 The Milky Way Galaxy (MWG . 19 4.4.1 Scale Height . 19 4.4.2 Magellanic Clouds . 20 5 Galaxy Interactions 20 6 Interstellar Medium 21 7 Active Galactic Nuclei 22 7.1 AGN Equations . 23 1 8 Spectra 25 8.1 21 cm line . 26 9 Black Holes 26 9.1 Stellar Black Holes . -
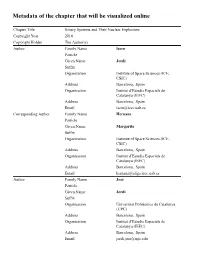
Metadata of the Chapter That Will Be Visualized Online
Metadata of the chapter that will be visualized online Chapter Title Binary Systems and Their Nuclear Explosions Copyright Year 2018 Copyright Holder The Author(s) Author Family Name Isern Particle Given Name Jordi Suffix Organization Institute of Space Sciences (ICE, CSIC) Address Barcelona, Spain Organization Institut d’Estudis Espacials de Catalunya (IEEC) Address Barcelona, Spain Email [email protected] Corresponding Author Family Name Hernanz Particle Given Name Margarita Suffix Organization Institute of Space Sciences (ICE, CSIC) Address Barcelona, Spain Organization Institut d’Estudis Espacials de Catalunya (IEEC) Address Barcelona, Spain Email [email protected] Author Family Name José Particle Given Name Jordi Suffix Organization Universitat Politècnica de Catalunya (UPC) Address Barcelona, Spain Organization Institut d’Estudis Espacials de Catalunya (IEEC) Address Barcelona, Spain Email [email protected] Abstract The nuclear energy supply of a typical star like the Sun would be ∼ 1052 erg if all the hydrogen could be incinerated into iron peak elements. Chapter 5 1 Binary Systems and Their Nuclear 2 Explosions 3 Jordi Isern, Margarita Hernanz, and Jordi José 4 5.1 Accretion onto Compact Objects and Thermonuclear 5 Runaways 6 The nuclear energy supply of a typical star like the Sun would be ∼1052 erg if all 7 the hydrogen could be incinerated into iron peak elements. Since the gravitational 8 binding energy is ∼1049 erg, it is evident that the nuclear energy content is more 9 than enough to blow up the Sun. However, stars are stable thanks to the fact that their 10 matter obeys the equation of state of a classical ideal gas that acts as a thermostat: if 11 some energy is released as a consequence of a thermal fluctuation, the gas expands, 12 the temperature drops and the instability is quenched. -

Cyclotron Radiation from Magnetic Cataclysmic Variables (Polarization, Plasmas, Magnetized, Stars, Herculis, Puppis)
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1985 Cyclotron Radiation From Magnetic Cataclysmic Variables (Polarization, Plasmas, Magnetized, Stars, Herculis, Puppis). Paul Everett aB rrett Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Barrett, Paul Everett, "Cyclotron Radiation From Magnetic Cataclysmic Variables (Polarization, Plasmas, Magnetized, Stars, Herculis, Puppis)." (1985). LSU Historical Dissertations and Theses. 4040. https://digitalcommons.lsu.edu/gradschool_disstheses/4040 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or “target” for pages apparently lacking from the document photographed is “ Missing Page(s)” . If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Stars and Their Spectra: an Introduction to the Spectral Sequence Second Edition James B
Cambridge University Press 978-0-521-89954-3 - Stars and Their Spectra: An Introduction to the Spectral Sequence Second Edition James B. Kaler Index More information Star index Stars are arranged by the Latin genitive of their constellation of residence, with other star names interspersed alphabetically. Within a constellation, Bayer Greek letters are given first, followed by Roman letters, Flamsteed numbers, variable stars arranged in traditional order (see Section 1.11), and then other names that take on genitive form. Stellar spectra are indicated by an asterisk. The best-known proper names have priority over their Greek-letter names. Spectra of the Sun and of nebulae are included as well. Abell 21 nucleus, see a Aurigae, see Capella Abell 78 nucleus, 327* ε Aurigae, 178, 186 Achernar, 9, 243, 264, 274 z Aurigae, 177, 186 Acrux, see Alpha Crucis Z Aurigae, 186, 269* Adhara, see Epsilon Canis Majoris AB Aurigae, 255 Albireo, 26 Alcor, 26, 177, 241, 243, 272* Barnard’s Star, 129–130, 131 Aldebaran, 9, 27, 80*, 163, 165 Betelgeuse, 2, 9, 16, 18, 20, 73, 74*, 79, Algol, 20, 26, 176–177, 271*, 333, 366 80*, 88, 104–105, 106*, 110*, 113, Altair, 9, 236, 241, 250 115, 118, 122, 187, 216, 264 a Andromedae, 273, 273* image of, 114 b Andromedae, 164 BDþ284211, 285* g Andromedae, 26 Bl 253* u Andromedae A, 218* a Boo¨tis, see Arcturus u Andromedae B, 109* g Boo¨tis, 243 Z Andromedae, 337 Z Boo¨tis, 185 Antares, 10, 73, 104–105, 113, 115, 118, l Boo¨tis, 254, 280, 314 122, 174* s Boo¨tis, 218* 53 Aquarii A, 195 53 Aquarii B, 195 T Camelopardalis, -

Jeremiah P. Ostriker
JEREMIAH P. OSTRIKER Section I: Curriculum Vitae Born: New York, New York, April 13, 1937 A.B. in Physics and Chemistry, Harvard, 1959 Ph.D. in Astrophysics, University of Chicago, 1964 H.D., Doctor of Science, University of Chicago, 1992 Master of Arts, University of Cambridge (England), 2002 Postdoctoral Fellow, University of Cambridge (England), 1964-65 Research Associate and Lecturer, Princeton University, 1965-66 Assistant Professor, Princeton University, 1966-68 Associate Professor, Princeton University, 1968-71 Professor, Princeton University, 1971- Chairman, Department of Astrophysical Sciences, Princeton University and Director, Princeton University Observatory, 1979-1995 Charles A. Young Professor of Astronomy, Princeton University, 1982-2002 Member of the Editorial Board and Trustee, Princeton University Press, 1982-84, 1986 Visiting Professor, Harvard University, and Regents Fellow, Smithsonian Institute, 1984-85, Regents Fellow 1987 Visiting Miller Professor, University of California-Berkeley, 1990 Provost, Princeton University, 1995-2001 American Museum of Natural History, Trustee, 1997-2006; Honorary Trustee, 2007-present Plumian Professor of Astronomy & Experimental Philosophy, IoA, Univ. of Cambridge (UK) 2001-2004 Distinguished Visitor, Institute for Advanced Study, 2004-2007 Director, Princeton Institute for Computational Science and Engineering, Princeton University, 2005-present Treasurer, National Academy of Sciences, July 2008-2012 Awards, Prizes and Fellowships National Science Foundation Fellowship, 1960-65 Alfred P. Sloan Fellowship, 1970-72 Helen B. Warner Prize of the American Astronomical Society, 1972 Sherman Fairchild Fellowship of California Institute of Technology, 1977 Henry Norris Russell Prize of the American Astronomical Society, 1980 Smithsonian Institution's Regents Fellowship, 1985 Fellow of the American Association for the Advancement of Science, 1992 Vainu Bappu Memorial Award of the Indian National Science Academy, 1993 Karl Schwarzschild Medal of the Astronomische Gesellschaft, 1999 U. -

Keith Horne: Refereed Publications Papers Submitted: 425. “A
Keith Horne: Refereed Publications Papers Submitted: 427. “The Lick AGN Monitoring Project 2016: Velocity-Resolved Hβ Lags in Luminous Seyfert Galaxies.” V.U, A.J.Barth, H.A.Vogler, H.Guo, T.Treu, et al. (202?). ApJ, submitted (01 Oct 2021). 426. “Multi-wavelength Optical and NIR Variability Analysis of the Blazar PKS 0027-426.” E.Guise, S.F.H¨onig, T.Almeyda, K.Horne M.Kishimoto, et al. (202?). (arXiv:2108.13386) 425. “A second planet transiting LTT 1445A and a determination of the masses of both worlds.” J.G.Winters, et al. (202?) ApJ, submitted (30 Jul 2021). (arXiv:2107.14737) 424. “A Different-Twin Pair of Sub-Neptunes orbiting TOI-1064 Discovered by TESS, Characterised by CHEOPS and HARPS” T.G.Wilson et al. (202?). ApJ, submitted (12 Jul 2021). 423. “The LHS 1678 System: Two Earth-Sized Transiting Planets and an Astrometric Companion Orbiting an M Dwarf Near the Convective Boundary at 20 pc” M.L.Silverstein, et al. (202?). AJ, submitted (24 Jun 2021). 422. “A temperate Earth-sized planet with strongly tidally-heated interior transiting the M8 dwarf LP 791-18.” M.Peterson, B.Benneke, et al. (202?). submitted (09 May 2021). 421. “The Sloan Digital Sky Survey Reverberation Mapping Project: UV-Optical Accretion Disk Measurements with Hubble Space Telescope.” Y.Homayouni, M.R.Sturm, J.R.Trump, K.Horne, C.J.Grier, Y.Shen, et al. (202?). ApJ submitted (06 May 2021). (arXiv:2105.02884) Papers in Press: 420. “Bayesian Analysis of Quasar Lightcurves with a Running Optimal Average: New Time Delay measurements of COSMOGRAIL Gravitationally Lensed Quasars.” F.R.Donnan, K.Horne, J.V.Hernandez Santisteban (202?) MNRAS, in press (28 Sep 2021). -

Publications of the Astronomical Society of the Pacific Vol. 106 1994 March No. 697 Publications of the Astronomical Society Of
Publications of the Astronomical Society of the Pacific Vol. 106 1994 March No. 697 Publications of the Astronomical Society of the Pacific 106: 209-238, 1994 March Invited Review Paper The DQ Herculis Stars Joseph Patterson Department of Astronomy, Columbia University, 538 West 120th Street, New York, New York 10027 Electronic mail: [email protected] Received 1993 September 2; accepted 1993 December 9 ABSTRACT. We review the properties of the DQ Herculis stars: cataclysmic variables containing an accreting, magnetic, rapidly rotating white dwarf. These stars are characterized by strong X-ray emission, high-excitation spectra, and very stable optical and X-ray pulsations in their light curves. There is considerable resemblance to their more famous cousins, the AM Herculis stars, but the latter class is additionally characterized by spin-orbit synchronism and the presence of strong circular polarization. We list eighteen stars passing muster as certain or very likely DQ Her stars. The rotational periods range from 33 s to 2.0 hr. Additional periods can result when the rotating searchlight illuminates other structures in the binary. A single hypothesis explains most of the observed properties: magnetically channeled accretion within a truncated disk. Some accretion flow still seems to proceed directly to the magnetosphere, however. The white dwarfs' magnetic moments are in the range 1032-1034 G cm3, slightly weaker than in AM Her stars but with some probable overlap. The more important reason why DQ Hers have broken synchronism is probably their greater accretion rate and orbital separation. The observed Lx/L v values are surprisingly low for a radially accreting white dwarf, suggesting that most of the accretion energy is not radiated in a strong shock above the magnetic pole. -

N95- 27078 13 Discussion on Selected Symbiotic Stars
N95- 27078 13 DISCUSSION ON SELECTED SYMBIOTIC STARS R. Viotti and M. Hack I. INTRODUCTION made of its variations" (Mayall, 1969). This pessimistic remark should be consid- Because of its large variety of aspects, the ered as a note of caution for those involved in symbiotic phenomenon is not very suitable for the interpretation of the observations. In the a statistical treatment. It is also not clear following, we shall discuss a number of indi- whether symbiotic stars really represent a vidual symbiotic stars for which the amount of homogeneous group of astrophysical objects or observational data is large enough to draw a a collection of objects of different natures but rather complete picture of their general behav- showing similar phenomena. However, as al- ior and to make consistent models. We shall ready discussed in the introduction to the sym- especially illustrate the necessary steps toward biotic stars, in this monograph we are espe- an empirical model and take the discussion of cially interested in the symbiotic phenomenon, the individual objects as a useful occasion to i.e., in those physical processes occurring in describe different techniques of diagnosis. the atmosphere of each individual object and in their time dependence. Such a research can be I1. Z ANDROMEDAE AND THE DIAGNOS- performed through the detailed analysis of TICS OF THE SYMBIOTIC STARS individual objects. This study should be done for a time long enough to cover all the different II.A. INTRODUCTION phases of their activity, in all the spectral ranges. Since the typical time scale of the symbiotic phenomena is up to several years and Z And has been considered as the prototype decades, this represents a problem since, for of the symbiotic stars, from its light history and instance, making astronomy outside the visual the spectral variation during outburst. -

Variable Star
Variable star A variable star is a star whose brightness as seen from Earth (its apparent magnitude) fluctuates. This variation may be caused by a change in emitted light or by something partly blocking the light, so variable stars are classified as either: Intrinsic variables, whose luminosity actually changes; for example, because the star periodically swells and shrinks. Extrinsic variables, whose apparent changes in brightness are due to changes in the amount of their light that can reach Earth; for example, because the star has an orbiting companion that sometimes Trifid Nebula contains Cepheid variable stars eclipses it. Many, possibly most, stars have at least some variation in luminosity: the energy output of our Sun, for example, varies by about 0.1% over an 11-year solar cycle.[1] Contents Discovery Detecting variability Variable star observations Interpretation of observations Nomenclature Classification Intrinsic variable stars Pulsating variable stars Eruptive variable stars Cataclysmic or explosive variable stars Extrinsic variable stars Rotating variable stars Eclipsing binaries Planetary transits See also References External links Discovery An ancient Egyptian calendar of lucky and unlucky days composed some 3,200 years ago may be the oldest preserved historical document of the discovery of a variable star, the eclipsing binary Algol.[2][3][4] Of the modern astronomers, the first variable star was identified in 1638 when Johannes Holwarda noticed that Omicron Ceti (later named Mira) pulsated in a cycle taking 11 months; the star had previously been described as a nova by David Fabricius in 1596. This discovery, combined with supernovae observed in 1572 and 1604, proved that the starry sky was not eternally invariable as Aristotle and other ancient philosophers had taught. -

Blast at Mrs
\‘ '■'i i -amLe M ■// t >, '• t - ,yl ' V. - y ~^ • , . \ X v ' - " ■ , ■ ■ ■ ^ ,'v ' ' ' - , r X ■J: ■)f PAGE TWENTY-Eii^ ,V":- )NESDAY, NOVEMBER 17, 1984 Stt^nins H^ralb ATflraga Dally Net Ptmb Run Far the Week Mnded The Covenant Laagua, young Nav. lli IfM rw w aet a? 0 & ^ a ^ m ■nstoM people of the Covenant Congrega About Town tional Church,' will held a rum mage sale'Saturday, Nov. 30, be 11,523 OeirtbHMd m ad. a w a la m l MgM U rt Sacred Haart Mothere Clr- tween the houn of •' a. m. and 2 Meaabair a( th a Audit rala toalght aa4 aariy Fldlijr. Laer Cl* win MMt tomorrow at 8 p. m. p. m. In the vacant store on Main Bureau a( Ofeuiatlaa .tauJghi near M. Bhawrare agate y ^ lIlh h lira. Jiamph J. Sylvester, 43 Street between ^ p le « a n d Eld- t b L Manchester-—‘A City of VUlage Charm Friday nigkt. Hlg« 8S-id. Scatborough Rd. ridge Streets. Miss Elsie C. John — "II................■------ son. ,123 Maple St., is chalrmi of the committee. VOL. LXXIV, NO. 42 -4— (TWENTY-EIGHT PAGES IN TWO SECTIONS) MANCHESTER, CONN., THURSDAY, NOVEMBER W, 1J54 (Ctoealfled aa Pugu N ) PRICE FIVE CENTS Temple Chapter No. 68^ Order of the Eastern Star, wtn open its HATS amiual fair tomorrow at 2 p. m. in the Masonic Tesriple. 'They will Reds, West Pair Cleared offer for sale a/Cholca selection of Seen aprons and irther gift articles, Of Charge in home made foods and candy, with ■/. Weigh New many bargains to be found on the '0 AEG "white elephant" table. -

Polarized Radiation from Inhomogeneous Shocks. Kinwah Wu Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1989 Polarized Radiation From Inhomogeneous Shocks. Kinwah Wu Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Wu, Kinwah, "Polarized Radiation From Inhomogeneous Shocks." (1989). LSU Historical Dissertations and Theses. 4825. https://digitalcommons.lsu.edu/gradschool_disstheses/4825 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps.