Answers to the Practical Questions and Problems Contained in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Observations from Orbiting Platforms 219
Dotto et al.: Observations from Orbiting Platforms 219 Observations from Orbiting Platforms E. Dotto Istituto Nazionale di Astrofisica Osservatorio Astronomico di Torino M. A. Barucci Observatoire de Paris T. G. Müller Max-Planck-Institut für Extraterrestrische Physik and ISO Data Centre A. D. Storrs Towson University P. Tanga Istituto Nazionale di Astrofisica Osservatorio Astronomico di Torino and Observatoire de Nice Orbiting platforms provide the opportunity to observe asteroids without limitation by Earth’s atmosphere. Several Earth-orbiting observatories have been successfully operated in the last decade, obtaining unique results on asteroid physical properties. These include the high-resolu- tion mapping of the surface of 4 Vesta and the first spectra of asteroids in the far-infrared wave- length range. In the near future other space platforms and orbiting observatories are planned. Some of them are particularly promising for asteroid science and should considerably improve our knowledge of the dynamical and physical properties of asteroids. 1. INTRODUCTION 1800 asteroids. The results have been widely presented and discussed in the IRAS Minor Planet Survey (Tedesco et al., In the last few decades the use of space platforms has 1992) and the Supplemental IRAS Minor Planet Survey opened up new frontiers in the study of physical properties (Tedesco et al., 2002). This survey has been very important of asteroids by overcoming the limits imposed by Earth’s in the new assessment of the asteroid population: The aster- atmosphere and taking advantage of the use of new tech- oid taxonomy by Barucci et al. (1987), its recent extension nologies. (Fulchignoni et al., 2000), and an extended study of the size Earth-orbiting satellites have the advantage of observing distribution of main-belt asteroids (Cellino et al., 1991) are out of the terrestrial atmosphere; this allows them to be in just a few examples of the impact factor of this survey. -

Tribute to an Astronomer: the Work of Max Ernst on Wilhelm Tempel
Tribute to an astronomer: the work of Max Ernst on Wilhelm Tempel Yaël Nazé (Research associate FNRS, ULg) Abstract In 1964-1974, the German artist Max Ernst created, with the help of two friends, a series of works (books, movie, paintings) related to the astronomer Wilhelm Tempel. Mixing actual texts by Tempel and artistic features, this series pays homage to the astronomer by recalling his life and discoveries. Moreover, the core of the project, the book Maximiliana or the Illegal Practice of Astronomy , actually depicts the way science works, making this work of art a most original tribute to a scientist. Keywords Wilhelm Tempel, Max Ernst, Art and Astronomy Art and the astronomer Amongst the many works of art related to astronomy, a subset is linked to astronomers themselves rather than celestial objects or theories 1. These paintings or sculptures fall in two broad categories. The first comprises portraits intended to represent specific people. The likeness to the actual scientist depicted varies greatly from work to work. Some portraits bear a close resemblance to the person represented – usually, these are contemporaneous and the artist actually met the scientist (e.g. Galileo by Justus Sustermans in 1636). Others have only a remote resemblance to the model, which usually happens when portraits are made well after the subject’s death. For example, the famous School of Athens by Raphaël, despite being a masterpiece, cannot be considered to show the actual faces of Plato, Aristotle, or Archimedes. In this context, plain errors are sometimes made: the portrait by Ford Madox Brown of William Crabtree Observing the Transit of Venus , for example, displays a man of great age while Crabtree was only 29 at the time of the event! The second category of astronomers’ portraits is composed of works depicting a generic figure unrelated to any specific scientist. -
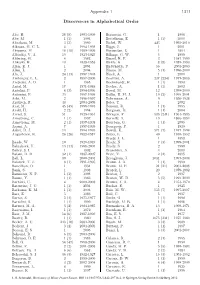
Appendix 1 1311 Discoverers in Alphabetical Order
Appendix 1 1311 Discoverers in Alphabetical Order Abe, H. 28 (8) 1993-1999 Bernstein, G. 1 1998 Abe, M. 1 (1) 1994 Bettelheim, E. 1 (1) 2000 Abraham, M. 3 (3) 1999 Bickel, W. 443 1995-2010 Aikman, G. C. L. 4 1994-1998 Biggs, J. 1 2001 Akiyama, M. 16 (10) 1989-1999 Bigourdan, G. 1 1894 Albitskij, V. A. 10 1923-1925 Billings, G. W. 6 1999 Aldering, G. 4 1982 Binzel, R. P. 3 1987-1990 Alikoski, H. 13 1938-1953 Birkle, K. 8 (8) 1989-1993 Allen, E. J. 1 2004 Birtwhistle, P. 56 2003-2009 Allen, L. 2 2004 Blasco, M. 5 (1) 1996-2000 Alu, J. 24 (13) 1987-1993 Block, A. 1 2000 Amburgey, L. L. 2 1997-2000 Boattini, A. 237 (224) 1977-2006 Andrews, A. D. 1 1965 Boehnhardt, H. 1 (1) 1993 Antal, M. 17 1971-1988 Boeker, A. 1 (1) 2002 Antolini, P. 4 (3) 1994-1996 Boeuf, M. 12 1998-2000 Antonini, P. 35 1997-1999 Boffin, H. M. J. 10 (2) 1999-2001 Aoki, M. 2 1996-1997 Bohrmann, A. 9 1936-1938 Apitzsch, R. 43 2004-2009 Boles, T. 1 2002 Arai, M. 45 (45) 1988-1991 Bonomi, R. 1 (1) 1995 Araki, H. 2 (2) 1994 Borgman, D. 1 (1) 2004 Arend, S. 51 1929-1961 B¨orngen, F. 535 (231) 1961-1995 Armstrong, C. 1 (1) 1997 Borrelly, A. 19 1866-1894 Armstrong, M. 2 (1) 1997-1998 Bourban, G. 1 (1) 2005 Asami, A. 7 1997-1999 Bourgeois, P. 1 1929 Asher, D. -

Asteroid Photometric and Polarimetric Phase Curves: Joint Linear-Exponential Modeling
Meteoritics & Planetary Science 44, Nr 12, 1937–1946 (2009) Abstract available online at http://meteoritics.org Asteroid photometric and polarimetric phase curves: Joint linear-exponential modeling K. MUINONEN1, 2, A. PENTTILÄ1, A. CELLINO3, I. N. BELSKAYA4, M. DELBÒ5, A. C. LEVASSEUR-REGOURD6, and E. F. TEDESCO7 1University of Helsinki, Observatory, Kopernikuksentie 1, P.O. BOX 14, FI-00014 U. Helsinki, Finland 2Finnish Geodetic Institute, Geodeetinrinne 2, P.O. Box 15, FI-02431 Masala, Finland 3INAF-Osservatorio Astronomico di Torino, strada Osservatorio 20, 10025 Pino Torinese, Italy 4Astronomical Institute of Kharkiv National University, 35 Sumska Street, 61035 Kharkiv, Ukraine 5IUMR 6202 Laboratoire Cassiopée, Observatoire de la Côte d’Azur, BP 4229, 06304 Nice, Cedex 4, France 6UPMC Univ. Paris 06, UMR 7620, BP3, 91371 Verrières, France 7Planetary Science Institute, 1700 E. Ft. Lowell Road, Tucson, Arizona 85719, USA *Corresponding author. E-mail: [email protected] (Received 01 April, 2009; revision accepted 18 August 2009) Abstract–We present Markov-Chain Monte-Carlo methods (MCMC) for the derivation of empirical model parameters for photometric and polarimetric phase curves of asteroids. Here we model the two phase curves jointly at phase angles գ25° using a linear-exponential model, accounting for the opposition effect in disk-integrated brightness and the negative branch in the degree of linear polarization. We apply the MCMC methods to V-band phase curves of asteroids 419 Aurelia (taxonomic class F), 24 Themis (C), 1 Ceres (G), 20 Massalia (S), 55 Pandora (M), and 64 Angelina (E). We show that the photometric and polarimetric phase curves can be described using a common nonlinear parameter for the angular widths of the opposition effect and negative-polarization branch, thus supporting the hypothesis of common physical mechanisms being responsible for the phenomena. -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

The Ch-‐Class Asteroids
The Ch-class asteroids: Connecting a visible taxonomic class to a 3-µm band shape Andrew S. Rivkin1*, Cristina A. Thomas2+, Ellen S. Howell3*^, Joshua P. Emery4* 1. The Johns Hopkins University Applied Physics Laboratory 2. NASA Goddard Space Flight Center, 3. Arecibo Observatory, USRA 4. University of Tennessee Accepted to The Astronomical Journal 3 November 2015 *Visiting Astronomer at the Infrared Telescope Facility, which is operated by the University of Hawaii under Cooperative Agreement no. NNX-08AE38A with the National Aeronautics and Space Administration, Science Mission Directorate, Planetary Astronomy Program. +Currently also at the Planetary Science Institute. Work done while supported by an appointment to the NASA Postdoctoral Program, administered by Oak Ridge Associated Universities through a contract with NASA. ^Currently at Lunar and Planetary Laboratory, University of Arizona, Tucson AZ Abstract Asteroids belonging to the Ch spectral taxonomic class are defined by the presence of an absorption near 0.7 μm, which is interpreted as due to Fe-bearing phyllosilicates. Phyllosilicates also cause strong absorptions in the 3-μm region, as do other hydrated and hydroxylated minerals and H2O ice. Over the past decade, spectral observations have revealed different 3-µm band shapes the asteroid population. Although a formal taxonomy is yet to be fully established, the “Pallas-type” spectral group is most consistent with the presence of phyllosilicates. If Ch class and Pallas type are both indicative of phyllosilicates, then all Ch-class asteroids should also be Pallas-type. In order to test this hypothesis, we obtained 42 observations of 36 Ch-class asteroids in the 2- to 4-µm spectral region. -

Asteroids Do Have Satellites
Asteroids Do Have Satellites William J. Merline Southwest Research Institute (Boulder) Stuart J. Weidenschilling Planetary Science Institute Daniel D. Durda Southwest Research Institute (Boulder) Jean-Luc Margot California Institute of Technology Petr Pravec Astronomical Institute AS CR, Ondrejoˇ v, Czech Republic and Alex D. Storrs Towson University After years of speculation, satellites of asteroids have now been shown definitively to exist. Asteroid satellites are important in at least two ways: (1) they are a natural laboratory in which to study collisions, a ubiquitous and critically important process in the formation and evolution of the asteroids and in shaping much of the solar system, and (2) their presence allows to us to determine the density of the primary asteroid, something which otherwise (except for certain large asteroids that may have measurable gravitational influence on, e.g., Mars) would require a spacecraft flyby, orbital mission, or sample return. Satellites or binaries have now been detected in a variety of dynamical populations, including near- Earth, Main Belt, outer Main-Belt, Trojan, and trans-Neptunian. Detection of these new systems has been the result of improved observational techniques, including adaptive op- tics on large telescopes, radar, direct imaging, advanced lightcurve analysis, and spacecraft imaging. Systematics and differences among the observed systems give clues to the for- mation mechanisms. We describe several processes that may result in binary systems, all of which involve collisions of one type or another, either physical or gravitational. Several mechanisms will likely be required to explain the observations. 1 1 INTRODUCTION 1.1 Overview Discovery and study of small satellites of asteroids or double asteroids can yield valuable infor- mation about the intrinsic properties of asteroids themselves and about their history and evolution. -

A Radar Survey of Main-Belt Asteroids: Arecibo Observations of 55 Objects During 1999–2003
Icarus 186 (2007) 126–151 www.elsevier.com/locate/icarus A radar survey of main-belt asteroids: Arecibo observations of 55 objects during 1999–2003 Christopher Magri a,∗, Michael C. Nolan b,StevenJ.Ostroc, Jon D. Giorgini d a University of Maine at Farmington, 173 High Street—Preble Hall, Farmington, ME 04938, USA b Arecibo Observatory, HC3 Box 53995, Arecibo, PR 00612, USA c 300-233, Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91109-8099, USA d 301-150, Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91109-8099, USA Received 3 June 2006; revised 10 August 2006 Available online 24 October 2006 Abstract We report Arecibo observations of 55 main-belt asteroids (MBAs) during 1999–2003. Most of our targets had not been detected previously with radar, so these observations more than double the number of radar-detected MBAs. Our bandwidth estimates constrain our targets’ pole directions in a manner that is geometrically distinct from optically derived constraints. We present detailed statistical analyses of the disk-integrated properties (radar albedo and circular polarization ratio) of the 84 MBAs observed with radar through March 2003; all of these observations are summarized in the online supplementary information. Certain conclusions reached in previous studies are strengthened: M asteroids have higher mean radar albedos and a wider range of albedos than do other MBAs, suggesting that both metal-rich and metal-poor M-class objects exist; and C- and S-class MBAs have indistinguishable radar albedo distributions, suggesting that most S-class objects are chondritic. Also in accord with earlier results, there is evidence that primitive asteroids from outside the C taxon (F, G, P, and D) are not as radar-bright as C and S objects, but a convincing statistical test must await larger sample sizes. -
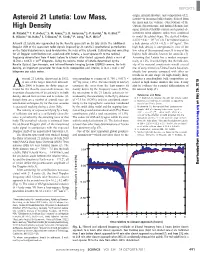
Asteroid 21 Lutetia: Low Mass, Lutetia—Is Its Mean (Bulk) Density, Derived from the Mass and the Volume
REPORTS origin, internal structure, and composition of 21 Asteroid 21 Lutetia: Low Mass, Lutetia—is its mean (bulk) density, derived from the mass and the volume. Observations of the High Density Optical, Spectroscopic, and Infrared Remote Im- aging System (OSIRIS) camera and ground ob- M. Pätzold,1* T. P. Andert,2 S. W. Asmar,3 J. D. Anderson,3 J.-P. Barriot,4 M. K. Bird,1,5 servations using adaptive optics were combined B. Häusler,2 M. Hahn,1 S. Tellmann,1 H. Sierks,6 P. Lamy,7 B. P. Weiss8 to model the global shape. The derived volume is (5.0 T 0.4) × 1014 m3 (4). The volume leads to Asteroid 21 Lutetia was approached by the Rosetta spacecraft on 10 July 2010. The additional a bulk density of (3.4 T 0.3) × 103 kg/m3.This Doppler shift of the spacecraft radio signals imposed by 21 Lutetia’s gravitational perturbation high bulk density is unexpected in view of the on the flyby trajectory were used to determine the mass of the asteroid. Calibrating and correcting low value of the measured mass. It is one of the for all Doppler contributions not associated with Lutetia, a least-squares fit to the residual highest bulk densities known for asteroids (5). frequency observations from 4 hours before to 6 hours after closest approach yields a mass of Assuming that Lutetia has a modest macropo- (1.700 T 0.017) × 1018 kilograms. Using the volume model of Lutetia determined by the rosity of 12%, it would imply that the bulk den- Rosetta Optical, Spectroscopic, and Infrared Remote Imaging System (OSIRIS) camera, the bulk sity of its material constituents would exceed density, an important parameter for clues to its composition and interior, is (3.4 T 0.3) × 103 that of stony meteorites. -

Nasa Technical Memorandum .1
NASA TECHNICAL MEMORANDUM NASA TM X-64677 COMETS AND ASTEROIDS: A Strategy for Exploration REPORT OF THE COMET AND ASTEROID MISSION STUDY PANEL May 1972 .1!vP -V (NASA-TE-X- 6 q767 ) COMETS AND ASTEROIDS: A RR EXPLORATION (NASA) May 1972 CSCL 03A NATIONAL AERONAUTICS AND SPACE ADMINISTRATION Reproduced by ' NATIONAL "TECHINICAL INFORMATION: SERVICE US Depdrtmett ofCommerce :. Springfield VA 22151 TECHNICAL REPORT STANDARD TITLE PAGE · REPORT NO. 2. GOVERNMENT ACCESSION NO. 3, RECIPIENT'S CATALOG NO. NASA TM X-64677 . TITLE AND SUBTITLE 5. REPORT aE COMETS AND ASTEROIDS ___1_ A Strategy for Exploration 6. PERFORMING ORGANIZATION CODE AUTHOR(S) 8. PERFORMING ORGANIZATION REPORT # Comet and Asteroid Mission Study Panel PERFORMING ORGANIZATION NAME AND ADDRESS 10. WORK UNIT NO. 11. CONTRACT OR GRANT NO. 13. TYPE OF REPORT & PERIOD COVERED 2. SPONSORING AGENCY NAME AND ADDRESS National Aeronautics and Space Administration Technical Memorandum Washington, D. C. 20546 14. SPONSORING AGENCY CODE 5. SUPPLEMENTARY NOTES ABSTRACT Many of the asteroids probably formed near the orbits where they are found today. They accreted from gases and particles that represented the primordial solar system cloud at that location. Comets, in contrast to asteroids, probably formed far out in the solar system, and at very low temperatures; since they have retained their volatile components they are probably the most primordial matter that presently can be found anywhere in the solar system. Exploration and detailed study of comets and asteroids, therefore, should be a significant part of NASA's efforts to understand the solar system. A comet and asteroid program should consist of six major types of projects: ground-based observations;Earth-orbital observations; flybys; rendezvous; landings; and sample returns. -
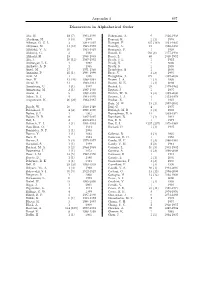
Appendix 1 897 Discoverers in Alphabetical Order
Appendix 1 897 Discoverers in Alphabetical Order Abe, H. 22 (7) 1993-1999 Bohrmann, A. 9 1936-1938 Abraham, M. 3 (3) 1999 Bonomi, R. 1 (1) 1995 Aikman, G. C. L. 3 1994-1997 B¨orngen, F. 437 (161) 1961-1995 Akiyama, M. 14 (10) 1989-1999 Borrelly, A. 19 1866-1894 Albitskij, V. A. 10 1923-1925 Bourgeois, P. 1 1929 Aldering, G. 3 1982 Bowell, E. 563 (6) 1977-1994 Alikoski, H. 13 1938-1953 Boyer, L. 40 1930-1952 Alu, J. 20 (11) 1987-1993 Brady, J. L. 1 1952 Amburgey, L. L. 1 1997 Brady, N. 1 2000 Andrews, A. D. 1 1965 Brady, S. 1 1999 Antal, M. 17 1971-1988 Brandeker, A. 1 2000 Antonini, P. 25 (1) 1996-1999 Brcic, V. 2 (2) 1995 Aoki, M. 1 1996 Broughton, J. 179 1997-2002 Arai, M. 43 (43) 1988-1991 Brown, J. A. 1 (1) 1990 Arend, S. 51 1929-1961 Brown, M. E. 1 (1) 2002 Armstrong, C. 1 (1) 1997 Broˇzek, L. 23 1979-1982 Armstrong, M. 2 (1) 1997-1998 Bruton, J. 1 1997 Asami, A. 5 1997-1999 Bruton, W. D. 2 (2) 1999-2000 Asher, D. J. 9 1994-1995 Bruwer, J. A. 4 1953-1970 Augustesen, K. 26 (26) 1982-1987 Buchar, E. 1 1925 Buie, M. W. 13 (1) 1997-2001 Baade, W. 10 1920-1949 Buil, C. 4 1997 Babiakov´a, U. 4 (4) 1998-2000 Burleigh, M. R. 1 (1) 1998 Bailey, S. I. 1 1902 Burnasheva, B. A. 13 1969-1971 Balam, D. -

The Minor Planet Bulletin, We Feel Safe in Al., 1989)
THE MINOR PLANET BULLETIN OF THE MINOR PLANETS SECTION OF THE BULLETIN ASSOCIATION OF LUNAR AND PLANETARY OBSERVERS VOLUME 43, NUMBER 3, A.D. 2016 JULY-SEPTEMBER 199. PHOTOMETRIC OBSERVATIONS OF ASTEROIDS star, and asteroid were determined by measuring a 5x5 pixel 3829 GUNMA, 6173 JIMWESTPHAL, AND sample centered on the asteroid or star. This corresponds to a 9.75 (41588) 2000 SC46 by 9.75 arcsec box centered upon the object. When possible, the same comparison star and check star were used on consecutive Kenneth Zeigler nights of observation. The coordinates of the asteroid were George West High School obtained from the online Lowell Asteroid Services (2016). To 1013 Houston Street compensate for the effect on the asteroid’s visual magnitude due to George West, TX 78022 USA ever changing distances from the Sun and Earth, Eq. 1 was used to [email protected] vertically align the photometric data points from different nights when constructing the composite lightcurve: Bryce Hanshaw 2 2 2 2 George West High School Δmag = –2.5 log((E2 /E1 ) (r2 /r1 )) (1) George West, TX USA where Δm is the magnitude correction between night 1 and 2, E1 (Received: 2016 April 5 Revised: 2016 April 7) and E2 are the Earth-asteroid distances on nights 1 and 2, and r1 and r2 are the Sun-asteroid distances on nights 1 and 2. CCD photometric observations of three main-belt 3829 Gunma was observed on 2016 March 3-5. Weather asteroids conducted from the George West ISD Mobile conditions on March 3 and 5 were not particularly favorable and so Observatory are described.