A. Introduction B. Magnification, Resolution, and Working Distance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Galileo and the Telescope
Galileo and the Telescope A Discussion of Galileo Galilei and the Beginning of Modern Observational Astronomy ___________________________ Billy Teets, Ph.D. Acting Director and Outreach Astronomer, Vanderbilt University Dyer Observatory Tuesday, October 20, 2020 Image Credit: Giuseppe Bertini General Outline • Telescopes/Galileo’s Telescopes • Observations of the Moon • Observations of Jupiter • Observations of Other Planets • The Milky Way • Sunspots Brief History of the Telescope – Hans Lippershey • Dutch Spectacle Maker • Invention credited to Hans Lippershey (c. 1608 - refracting telescope) • Late 1608 – Dutch gov’t: “ a device by means of which all things at a very great distance can be seen as if they were nearby” • Is said he observed two children playing with lenses • Patent not awarded Image Source: Wikipedia Galileo and the Telescope • Created his own – 3x magnification. • Similar to what was peddled in Europe. • Learned magnification depended on the ratio of lens focal lengths. • Had to learn to grind his own lenses. Image Source: Britannica.com Image Source: Wikipedia Refracting Telescopes Bend Light Refracting Telescopes Chromatic Aberration Chromatic aberration limits ability to distinguish details Dealing with Chromatic Aberration - Stop Down Aperture Galileo used cardboard rings to limit aperture – Results were dimmer views but less chromatic aberration Galileo and the Telescope • Created his own (3x, 8-9x, 20x, etc.) • Noted by many for its military advantages August 1609 Galileo and the Telescope • First observed the -

Compound Light Microscopes Magnification
Compound Light Microscopes • Frequently used tools of biologists. • Magnify organisms too small to be seen with the unaided eye. • To use: – Sandwich specimen between transparent slide and thin, transparent coverslip. – Shine light through specimen into lenses of microscope. • Lens closest to object is objective lens. • Lens closest to your eye is the ocular lens. • The image viewed through a compound light microscope is formed by the projection of light through a mounted specimen on a slide. Eyepiece/ ocular lens Magnification Nosepiece Arm Objectives/ • Magnification - the process of objective lens enlarging something in appearance, not Stage Clips Light intensity knob actual physical size. Stage Coarse DiaphragmDiaphragm Adjustment Fine Adjustment Light Positioning knobs Source Base Always carry a microscope with one hand holding the arm and one hand under the base. What’s my power? Comparing Powers of Magnification To calculate the power of magnification or total magnification, multiply the power of the ocular lens by the We can see better details with higher power of the objective. the powers of magnification, but we cannot see as much of the image. Which of these images would be viewed at a higher power of magnification? 1 Resolution Limit of resolution • Resolution - the shortest distance • As magnifying power increases, we see between two points more detail. on a specimen that • There is a point where we can see no can still be more detail is the limit of resolution. distinguished as – Beyond the limit of resolution, objects get two points. blurry and detail is lost. – Use electron microscopes to reveal detail beyond the limit of resolution of a compound light microscope! Proper handling technique Field of view 1. -

Lab 11: the Compound Microscope
OPTI 202L - Geometrical and Instrumental Optics Lab 9-1 LAB 9: THE COMPOUND MICROSCOPE The microscope is a widely used optical instrument. In its simplest form, it consists of two lenses Fig. 9.1. An objective forms a real inverted image of an object, which is a finite distance in front of the lens. This image in turn becomes the object for the ocular, or eyepiece. The eyepiece forms the final image which is virtual, and magnified. The overall magnification is the product of the individual magnifications of the objective and the eyepiece. Figure 9.1. Images in a compound microscope. To illustrate the concept, use a 38 mm focal length lens (KPX079) as the objective, and a 50 mm focal length lens (KBX052) as the eyepiece. Set them up on the optical rail and adjust them until you see an inverted and magnified image of an illuminated object. Note the intermediate real image by inserting a piece of paper between the lenses. Q1 ● Can you demonstrate the final image by holding a piece of paper behind the eyepiece? Why or why not? The eyepiece functions as a magnifying glass, or simple magnifier. In effect, your eye looks into the eyepiece, and in turn the eyepiece looks into the optical system--be it a compound microscope, a spotting scope, telescope, or binocular. In all cases, the eyepiece doesn't view an actual object, but rather some intermediate image formed by the "front" part of the optical system. With telescopes, this intermediate image may be real or virtual. With the compound microscope, this intermediate image is real, formed by the objective lens. -

Depth of Focus (DOF)
Erect Image Depth of Focus (DOF) unit: mm Also known as ‘depth of field’, this is the distance (measured in the An image in which the orientations of left, right, top, bottom and direction of the optical axis) between the two planes which define the moving directions are the same as those of a workpiece on the limits of acceptable image sharpness when the microscope is focused workstage. PG on an object. As the numerical aperture (NA) increases, the depth of 46 focus becomes shallower, as shown by the expression below: λ DOF = λ = 0.55µm is often used as the reference wavelength 2·(NA)2 Field number (FN), real field of view, and monitor display magnification unit: mm Example: For an M Plan Apo 100X lens (NA = 0.7) The depth of focus of this objective is The observation range of the sample surface is determined by the diameter of the eyepiece’s field stop. The value of this diameter in 0.55µm = 0.6µm 2 x 0.72 millimeters is called the field number (FN). In contrast, the real field of view is the range on the workpiece surface when actually magnified and observed with the objective lens. Bright-field Illumination and Dark-field Illumination The real field of view can be calculated with the following formula: In brightfield illumination a full cone of light is focused by the objective on the specimen surface. This is the normal mode of viewing with an (1) The range of the workpiece that can be observed with the optical microscope. With darkfield illumination, the inner area of the microscope (diameter) light cone is blocked so that the surface is only illuminated by light FN of eyepiece Real field of view = from an oblique angle. -

The Microscope Parts And
The Microscope Parts and Use Name:_______________________ Period:______ Historians credit the invention of the compound microscope to the Dutch spectacle maker, Zacharias Janssen, around the year 1590. The compound microscope uses lenses and light to enlarge the image and is also called an optical or light microscope (vs./ an electron microscope). The simplest optical microscope is the magnifying glass and is good to about ten times (10X) magnification. The compound microscope has two systems of lenses for greater magnification, 1) the ocular, or eyepiece lens that one looks into and 2) the objective lens, or the lens closest to the object. Before purchasing or using a microscope, it is important to know the functions of each part. Eyepiece Lens: the lens at the top that you look through. They are usually 10X or 15X power. Tube: Connects the eyepiece to the objective lenses Arm: Supports the tube and connects it to the base. It is used along with the base to carry the microscope Base: The bottom of the microscope, used for support Illuminator: A steady light source (110 volts) used in place of a mirror. Stage: The flat platform where you place your slides. Stage clips hold the slides in place. Revolving Nosepiece or Turret: This is the part that holds two or more objective lenses and can be rotated to easily change power. Objective Lenses: Usually you will find 3 or 4 objective lenses on a microscope. They almost always consist of 4X, 10X, 40X and 100X powers. When coupled with a 10X (most common) eyepiece lens, we get total magnifications of 40X (4X times 10X), 100X , 400X and 1000X. -

How Do the Lenses in a Microscope Work?
Student Name: _____________________________ Date: _________________ How do the lenses in a microscope work? Compound Light Microscope: A compound light microscope uses light to transmit an image to your eye. Compound deals with the microscope having more than one lens. Microscope is the combination of two words; "micro" meaning small and "scope" meaning view. Early microscopes, like Leeuwenhoek's, were called simple because they only had one lens. Simple scopes work like magnifying glasses that you have seen and/or used. These early microscopes had limitations to the amount of magnification no matter how they were constructed. The creation of the compound microscope by the Janssens helped to advance the field of microbiology light years ahead of where it had been only just a few years earlier. The Janssens added a second lens to magnify the image of the primary (or first) lens. Simple light microscopes of the past could magnify an object to 266X as in the case of Leeuwenhoek's microscope. Modern compound light microscopes, under optimal conditions, can magnify an object from 1000X to 2000X (times) the specimens original diameter. "The Compound Light Microscope." The Compound Light Microscope. Web. 16 Feb. 2017. http://www.cas.miamioh.edu/mbi-ws/microscopes/compoundscope.html Text is available under the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) license. - 1 – Student Name: _____________________________ Date: _________________ Now we will describe how a microscope works in somewhat more detail. The first lens of a microscope is the one closest to the object being examined and, for this reason, is called the objective. -

Scope & Spotting Scope Resolution and Magnification
Scope & Spotting Scope Resolution and Magnification Eyesight Resolution Limits The normal or median human visual resolution limit is 1 MOA (minute of angle). This is also called normal visual acuity when use by your eye doctor. On the typical Snellen eye chart, the 20/20 line (20 foot line in USA) or 6/6 line (6 meters in the rest of the world) was designed to be readable with a visual resolution or acuity of 1 MOA. The other lines/characters are scaled accordingly. Telescopes were developed as visual aids to overcome this limited visual resolution or acuity. The principal tool for resolution improvement is magnification. It is obvious that magnification increases the apparent size of objects. However and more importantly magnification increases visual resolution. In the following illustration note how even small (doublings – powers of 2) increases in magnification greatly increase our ability to resolve (distinguish) small but important details by both increasing their apparent size and making better use of the available field of view. Fred Bohl, 10 June 2007 Page 1 of 6 Scope & Spotting Scope Resolution and Magnification Scope Resolution Limits Diffraction Limited Optics A long historical record holds that diffraction defines the ultimate resolution limit of telescopes. Generally we can say that any aperture with a finite size will cause diffraction and hence its resolution will be limited. The finite aperture (front lens, main mirror) must cut off a part of the incoming plane wave front. This missing part is disturbing the otherwise perfect interference of the propagating waves in a certain way. The result is a modulation of the wave front called the Point Spread Function (PSF). -

THE STUDY of SATURN's RINGS 1 Thesis Presented for the Degree Of
1 THE STUDY OF SATURN'S RINGS 1610-1675, Thesis presented for the Degree of Doctor of Philosophy in the Field of History of Science by Albert Van Haden Department of History of Science and Technology Imperial College of Science and Teohnology University of London May, 1970 2 ABSTRACT Shortly after the publication of his Starry Messenger, Galileo observed the planet Saturn for the first time through a telescope. To his surprise he discovered that the planet does.not exhibit a single disc, as all other planets do, but rather a central disc flanked by two smaller ones. In the following years, Galileo found that Sa- turn sometimes also appears without these lateral discs, and at other times with handle-like appendages istead of round discs. These ap- pearances posed a great problem to scientists, and this problem was not solved until 1656, while the solution was not fully accepted until about 1670. This thesis traces the problem of Saturn, from its initial form- ulation, through the period of gathering information, to the final stage in which theories were proposed, ending with the acceptance of one of these theories: the ring-theory of Christiaan Huygens. Although the improvement of the telescope had great bearing on the problem of Saturn, and is dealt with to some extent, many other factors were in- volved in the solution of the problem. It was as much a perceptual problem as a technical problem of telescopes, and the mental processes that led Huygens to its solution were symptomatic of the state of science in the 1650's and would have been out of place and perhaps impossible before Descartes. -
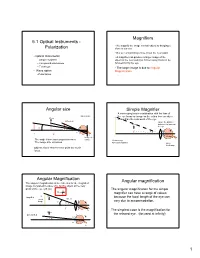
5.1 Optical Instruments - • We Magnify the Image a Small Object by Bringing It Polarization Close to Our Eye
Magnifiers 5.1 Optical Instruments - • We magnify the image a small object by bringing it Polarization close to our eye. • But we cannot bring it closer than the near point. • Optical Instruments • A magnifier can produce a larger image of the – Simple magnifier object at the near point (or farther away) that can be – Compound microscope focused on by the eye. – Telescope • The larger image is due to Angular • Wave optics Magnification –Polarization Angular size Simple Magnifier A converging lens in combination with the lens of Not to scale the eye forms an image on the retina from an object 25 cm closer than the near point of the eye. unfocused Ignore the distance 25 cm between the lens and the eye θ h P image on The angle θ increases as p decreases retina Virtual image The image size increases formed by magnifier Larger Real image Objects closer than the near point are not in focus. Angular Magnification Angular magnification The angular magnification is the ratio of θ for the magnified image compared to value of θo for the object at the near point of the eye. (25 cm) θ The angular magnification for the simple m = magnifier can have a range of values θo h’ 25 cm Magnified because the focal length of the eye can virtual object image vary due to accommodation. θ h • f The simplest case is the magnification for 25 cm Unmagnified the relaxed eye. (focused at infinity) h θo 1 Angular magnification Simple Magnifier 25 cm Simple magnifier. Magnified object θ h A simple magnifier with a focal length of 5.0 • h cm is used to view an insect. -

What's the Size of What You See? Determine the Field Diameter of a Compound Microscope
4th NGSS STEM Conference MAKING SCIENCE COUNT Integrating Math into an NGSS Classroom January 20, 2017 | Pier 15, San Francisco, CA What's the Size of What You See? Determine the field diameter of a compound microscope. By measuring the field diameter of a microscope, you can calculate the real sizes of objects too small to see with the naked eye. Tools and Materials • Compound microscope • Pencil and notepaper to record results • Calculator • Clear plastic metric ruler with millimeter markings (copy this template onto a transparency to make your own) • Optional: microscopic objects to view and measure 4th NGSS STEM Conference MAKING SCIENCE COUNT Integrating Math into an NGSS Classroom January 20, 2017 | Pier 15, San Francisco, CA Assembly None needed. To Do and Notice Find the total magnification of your microscope. First, read the power inscribed on the eyepiece. You’ll find it marked as a number followed by an X, which stands for “times.” Record the eyepiece power. Find the three barrel-shaped objective lenses near the microscope stage. Each will have a different power, which should be marked on the side of the lens. Record the power for each objective. Find the total magnification for each objective lens by multiplying the power of the eyepiece by the power of the objective. Lowest Magnification: Eyepiece x Lowest power objective = ________X Medium Magnification: Eyepiece x Medium power objective = ________X Highest Magnification: Eyepiece x Highest power objective = ________X Set the microscope to its lowest magnification. Slide the plastic metric ruler onto the stage and focus the microscope on the millimeter divisions. -

Is a Magnification of 20,000 Times Really Useful with Digital Microscopy?
Is a Magnification of 20,000 Times Really Useful With Digital Microscopy? Introduction Magnification defined Digital microscopes have only a camera for image observation Magnification is defined as the ratio of the size of a feature on and no eyepieces. Microscopes with eyepieces for visual an object as seen in an image to the actual size of the feature observation, such as stereo microscopes, can also be equipped itself. The lateral, two-dimensional magnification can be with digital cameras. Both types of microscope are used for a determined from: variety of technical applications in many different fields and Dimension of feature in image industries. Magnification = Dimension of feature on actual object To evaluate the performance of an optical microscope, knowing Examples of a digital microscope and stereo microscope with its highest achievable magnification is important. For digital eyepieces and digital camera are shown below. microscopy, very high magnification values, such as 20,000x, are sometimes mentioned. This report provides some helpful guidelines concerning the useful range of magnification for digital microscopy. Left: Leica M205 C stereo microscope fitted with the Leica DFC450 digital camera. The ant sample can be observed via the eyepieces or a display monitor (2 sizes shown) for image detection by the camera. Right: Leica DMS1000 digital microscope utilizing different monitor sizes for image display. Smaller monitor Camera chip Eyepieces Larger monitor Ant (sample) Left: Taxus, cross sectioned at –120 °C, investigated in a cryo SEM www.leica-microsystems.com USEFUL RANGE OF MAGNIFICATION FOR DIGITAL MICROSCOPY There is always the question if this level of magnification, Modern camera chips often have pixel sizes well below 10 µm 20,000 x, is simply beyond the useful range, meaning, is it and modern monitors pixel sizes well below 1 mm. -

Chapter 8 the Telescope
Chapter 8 The Telescope 8.1 Purpose In this lab, you will measure the focal lengths of two lenses and use them to construct a simple telescope which inverts the image like the one developed by Johannes Kepler. Because one lens has a large focal length and the other lens has a small focal length, you will use different methods of determining the focal lengths than was used in ’Optics of Thin Lenses’ lab. 8.2 Introduction 8.2.1 A Brief History of the Early Telescope Although eyeglass-makers had been experimenting with lenses well before 1600, the first mention of a telescope appears in a letter written in 1608 by Hans Lippershey, a Dutch spectacle maker, seeking a patent for a telescope. The patent was denied because of easy telescope duplication and difficulty in patent enforcement. The instrument spread rapidly. Galileo heard of it in the early 1600s and quickly made improvements in lens grinding that increased the magnification from a relatively low value of 2 to as much as 30. With these more powerful telescopes, he observed the Milky Way, the mountains on the Moon, the phases of Venus, and the moons of Jupiter. These early telescopes were a type of ‘opera glass,’ producing erect or ‘right side up’ images but having limited magnification. When Johannes Kepler, a German mathematician and astronomer working in Prague under Tycho Brahe, heard of Galileo’s discoveries, he perfected a different form of telescope. Although Kepler’s design inverts the image, it is much more powerful than the Galilean type. This lab we will use the lenses supplied with the telescope kit.