Medical Disposable Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
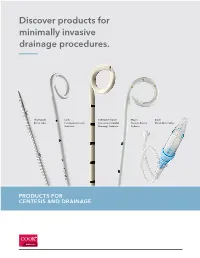
Discover Products for Minimally Invasive Drainage Procedures
Discover products for minimally invasive drainage procedures. Thal-Quick Lock Fuhrman Pleural/ Wayne Cook Chest Tube Pericardiocentesis Pneumopericardial Pneumothorax Chest Drain Valve Catheter Drainage Catheter Catheter MEDICAL Contents Pneumothorax catheters Wayne Pneumothorax Catheter Set and Tray – Seldinger ......................................................................................... 4 Wayne Pneumothorax Catheter Set – Trocar ............................................................................................................... 5 Cook Emergency Pneumothorax Set ........................................................................................................................... 6 Pneumothorax Set and Tray ........................................................................................................................................... 7 Richli Pneumothorax Catheter Set ................................................................................................................................ 8 Catheter Aspiration Set for Simple Pneumothorax .................................................................................................... 9 Multipurpose catheters Thal-Quick Chest Tube Set .......................................................................................................................................... 10 Thal-Quick Chest Tube Tray ......................................................................................................................................... 11 -

A Novel Wound and Soft Tissue Flap Negative Pressure Drain System - a Pilot Study Steven D
Archives of Health Science Research Article A Novel Wound and Soft Tissue Flap Negative Pressure Drain System - a Pilot Study Steven D. Jones Jr MD*1, Parker J. Prusick MD1, Bennie G. Lindeque MD PhD1 1Department of Orthopedic Surgery, University of Colorado Denver, 12631 E 17th Ave, Aurora, CO 80045, USA *Corresponding Author: Steven D. Jones Jr MD, Department of Orthopedic Surgery, University of Colorado Denver, 12631 E 17th Ave, Aurora, CO 80045, USA Abstract Background: Negative-pressure wound-therapy (NPWT) has become a mainstay of treatment for high-risk surgical wounds. In closed wounds, traditional NPWT utilizes surface level sponges alone to provide negative pressure. A technique that allows for deep dead-space management, while maintaining superficial negative pressure over a closed wound, may prove beneficial inhigh-risk patients. Purpose: A novel technique and prospective case series are described which incorporate deep hemovac drain tubings into a traditional NPWT device (Deep Inside-Out Vac; DIOV). Pilot data is needed to begin evaluating the efficacy of this technique. Methods: Fourteen patients were stratified by initial indication for DIOV placement. Group 1 patients underwent wide tumor resection, while Group 2 patients underwent extensive debridement for infection. Demographic, surgical, and microbiological data were recorded. Results: Eight patients were identified in Group 1. Six were identified in Group 2. Both demonstrated 50% positive culture rates at time of drain removal. Most common organisms were coagulase negative staphylococcus species. At final follow-up, all wounds were clinically healed. Conclusions: NPWT is an established augment in post-operative wound care. The DIOV may provide added benefit in wounds at high-risk for dead-space related complications. -

Wound Drain Tube Management
CLINICAL PROCEDURE WOUND DRAIN TUBE MANAGEMENT TARGET AUDIENCE All Peter Mac medical and nursing staff. STATE ANY RELATED PETER MAC POLICIES, PROCEDURES OR GUIDELINES Clinical Handover Policy Wound Management Guideline Hand Hygiene Procedure Aseptic Technique Procedure Care of Underwater Drainage Procedure Care of Percutaneous Nephrostomy Catheters Procedure Nursing Services Patient Health Assessment Guideline Patient Identification and Procedure Matching Procedure Observation and Response Chart Procedure PURPOSE This procedure aims to provide the target audience with best practice based evidence available, along with expert opinion, in regards to the management of drain tubes within the hospital setting. PROCEDURE Indication Drain tubes can be inserted prophylactically to either prevent or remove the accumulation of fluid in a wound. They can also be therapeutically inserted to evacuate an existing collection of fluid in a wound. Fluid is removed in order to treat or prevent infection and promote wound healing and patient comfort. Drain tubes can also be used to diagnose postoperative complications such as an anastomotic leak or haemorrhage. Types of Drainage Tubes ExudrainTM A closed, active drain system, with a negative pressure of approximately 75mmHg and a reservoir of 100mL. http://vitalmedikal.com.tr/yeni/index.php?option=com_content&task=view&id=9&Itemid=3 BellovacTM A closed, active drain system, with a negative pressure of approximately 90mmHg and a reservoir of 220mL. http://surgery.astratech.com.au/Main.aspx/Item/459337/navt/68686/navl/83954/nava/83974 Surimex Fixvac A closed, active drain system, with a negative pressure of approximately Vacuum System 338mmHg. It has a resevoir of 600mL. Please note: the bottle will only half fill and therefore will need to be changed when half filled. -

Answer Key Chapter 1
Instructor's Guide AC210610: Basic CPT/HCPCS Exercises Page 1 of 101 Answer Key Chapter 1 Introduction to Clinical Coding 1.1: Self-Assessment Exercise 1. The patient is seen as an outpatient for a bilateral mammogram. CPT Code: 77055-50 Note that the description for code 77055 is for a unilateral (one side) mammogram. 77056 is the correct code for a bilateral mammogram. Use of modifier -50 for bilateral is not appropriate when CPT code descriptions differentiate between unilateral and bilateral. 2. Physician performs a closed manipulation of a medial malleolus fracture—left ankle. CPT Code: 27766-LT The code represents an open treatment of the fracture, but the physician performed a closed manipulation. Correct code: 27762-LT 3. Surgeon performs a cystourethroscopy with dilation of a urethral stricture. CPT Code: 52341 The documentation states that it was a urethral stricture, but the CPT code identifies treatment of ureteral stricture. Correct code: 52281 4. The operative report states that the physician performed Strabismus surgery, requiring resection of the medial rectus muscle. CPT Code: 67314 The CPT code selection is for resection of one vertical muscle, but the medial rectus muscle is horizontal. Correct code: 67311 5. The chiropractor documents that he performed osteopathic manipulation on the neck and back (lumbar/thoracic). CPT Code: 98925 Note in the paragraph before code 98925, the body regions are identified. The neck would be the cervical region; the thoracic and lumbar regions are identified separately. Therefore, three body regions are identified. Correct code: 98926 Instructor's Guide AC210610: Basic CPT/HCPCS Exercises Page 2 of 101 6. -

Chronic Non Congestive Glaucoma: with Special Emphasis on Therapy
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1934 Chronic non congestive glaucoma: with special emphasis on therapy W. Morrison University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Morrison, W., "Chronic non congestive glaucoma: with special emphasis on therapy" (1934). MD Theses. 339. https://digitalcommons.unmc.edu/mdtheses/339 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. CHRONIC NON-CONGESTIVE GLAUCOMA: WITH ESPECIAL EMPHASIS ON THERAPY -. w. HOWARD MORRISON CHRONIC NON CONGESTIVE GLAUCOMA: with especial emphasis on therapy INTRODUCTION AND HISTORY It is my intention herein to discuss chronic non congestive glaucoma only briefly as an entirety, so that a suitable back ground may be constructed for the more exhaustive perusal of the recent literature on the therapy in that particular type of glaucoma. Throughout this paper the terms chronic non-congestive glaucoma, Simple glaucoma and glaucoma simplex will all refer to the same condition. The term glaucoma is not the title of anyone Single disease but is a clinical label for a complex of symptoms. Over four centuries before the Christian era, Hippocrates described glaukos as among the known affections of the eye. The Greek word, glaukos, he used to describe the disease because he saw a gray green reflex from the pupil. -

AST Guidelines for Counts
Recommended Standard of Practice for Counts Introduction The following Recommended Standards of Practice were researched and written by the AST Education and Professional Standards Committee and have been approved by the AST Board of Directors. They are effective October 27, 2006. AST developed the following Recommended Standards of Practice to support facilities in the reinforcement of best practices, related to performing the sponge, needle and instrument counts in the perioperative setting. The purpose of the Recommended Standards is to provide an outline that surgical team members can use to develop and implement policies and procedures for counts. The Recommended Standards is presented with the understanding that it is the responsibility of the healthcare facility to develop, approve, and establish policies and procedures for performing counts, according to established healthcare facility protocols. Rationale The following are Recommended Standards of Practice related to properly performing sponge, needle and instrument counts in the perioperative setting. It is recommended that sponge, needle and instrument counts be performed on all procedures that present with the possibility that a foreign object could be retained in order to increase patient safety practices in the perioperative setting. Risk factors identified as increasing the occurrence of an incorrect count or retained item include the following: emergency surgical procedures, unexpected change in the scope of the surgical procedure, procedures involving more than one surgical team, extended procedural length of time, unexpected transfusions, and morbidly obese patients.18 One of several safe patient outcomes related to surgery is all items viewed as retainable foreign objects are accounted for at the end of the surgical procedure, due to careful counting and documentation by the surgical team. -

Products for Emergency Medicine and Trauma
PRODUCTS FOR EMERGENCY MEDICINE AND TRAUMA MEDICAL Cook Medical offers a comprehensive selection of products for emergency medicine to aid in the resuscitation and treatment of your patients. Our devices have been engineered to provide you with minimally invasive solutions for the numerous issues critically ill or injured patients face, including airway obstruction, difficulty breathing, and loss of fluid. AIRWAY Airways are usually the initial focus in the resuscitation of a critically ill or injured patient, but sometimes conventional endotracheal intubation can be difficult or even impossible in individuals with a challenging anatomy or serious trauma. For these situations, Cook has developed a variety of emergency devices to help you obtain airway access. BREATHING Often the inability to maintain proper airway exchange may be a direct result of abnormal chest wall dynamics, such as a pneumothorax. Cook offers an extensive line of drainage catheters for removing both air and fluid from the pleural space. Available in straight and pigtail designs, these catheters are placed with either the Seldinger technique or by direct puncture. CIRCULATION Correcting circulation problems is another crucial step in emergency care, as the rapid replacement of fluid volume is imperative in the treatment of shock. Cook’s intraosseous infusion needles can be used to infuse drugs or fluids when intravenous access is not possible. Contents Difficult airway Intubation Frova Intubating Introducer ..................................................................................................................................... -

The Drainage of Subretinal Fluid: a Randomized Controlled Clinical Trial
THE DRAINAGE OF SUBRETINAL FLUID: A RANDOMIZED CONTROLLED CLINICAL TRIAL BY George F. Hilton, MD INTRODUCTION AMONG THE MANY CONTROVERSIES IN RETINAL DETACHMENT SURGERY, NONE HAS been more persistent than the unresolved question of drainage versus nondrainage. The controversy has persisted for over 20 years, and more than 60 papers have been written on the subject; however, to date there have been no controlled studies. The ongoing interest in this problem is illustrated by a recent Jules Gonin Club symposium on the drainage of subretinal fluid. After numer- ous papers on the subject the final summary acknowledged: "We are still challenged by the question: To drain or not to drain.'"1 The interest in this question is enhanced by the fact that most retinal surgeons regard the procedure ofsubretinal fluid drainage as a potentially hazardous step. Martin2 has defined it as "the most dangerous part of a retinal detachment operation." Ferguson3 referred to it as "the most crucial point" in the operation, and Norton4 observed that "fluid drainage is the one aspect ofthe surgical procedure over which the surgeon has the least control and although complications are unusual, they can be disas- trous." Not all authors are equally impressed with the potential complica- tions ofdrainage. Chawla5 regarded this surgical step as "only one danger among equals," and Schepens6 wrote that "the rate of complications from correctly performed perforation for the release of subretinal fluid is less than 1%." This question was recently brought into focus by a pair of papers representing the two major schools of thought on the issue. -

Surgical Products
Cervical & Endocervical Samplers Surgical Products Uterine Manipulation Devices Hysterectomy Instruments Laparoscopic Port-Site Closure Laparoscopic Instruments Laparoscopic Smoke Evacuation Laparoscopic Disposables Trocars Urology Instruments Retractors Delivering on the Promise to Help Clinicians Provide Better Health Care for Women Our mission is to enable physicians to provide increasingly more efficient and effective health care for women. We leverage our expertise in identifying and developing a steady stream of exceptional products that keep Ob/Gyns and others directly involved in caring for women, at the forefront of women’s health care. An Expansive Portfolio of Trusted Brands And Services Since our founding in 1990, CooperSurgical has researched, developed and manufactured a wide range of trusted brands that have advanced the standard of women’s health care. Our products are used by physicians and health care professionals in hospitals and their practices for a wide variety of procedures from basic annual gynecological exams to complex surgical procedures. As a full-service R&D and manufacturing company, we are committed to: • Improved patient outcomes and satisfaction • The highest quality and safety standards in the industry • Clinically proven technologies that provide ease of use • Technical support of our equipment – 20+ years of experience • Innovative solutions that reduce procedural costs • Annual support of medical professional educational organizations • Educational and in-service support of our products • Personalized service and a commitment to your satisfaction by educated professional representatives We appreciate the opportunity to partner with you to provide optimum health care for women. 2 To place an order, call us at: 800.243.2974 | Visit us online at: www.CooperSurgical.com About CooperSurgical Delivering on the Promise to Help Clinicians Provide Better Health Care for Women Industry-Leading Solutions for Laparoscopic and General Surgical Procedures CooperSurgical instruments are used every day in surgical procedures. -

Surgical Instruments
STANDARD SURGICAL INSTRUMENTS Section ''C'' Eye Ear Nasal Dermal Oral Tonsil Tracheal Laryngeal Esophageal Stomach Intestinal Gall Bladder ST ANDARD SURGICAL INSTRUMENTS C-10 C-30 C-40 - 3 Condensing Lenses, Illuminators, Ophthalmoscopes and Retinoscopes ·c- . <...onden ing Len~. 2 inches. hard rubber rim, 13 dtoptar. ·c ond n mg Len . double. 1:3 and dioptar. ·c- 1 ond n 'ng Len • \\ith metal rim and handle. ·c Dr . Berger's upc ''ith met I hea band. ·c- 9 Dr. Bez:ger's upe with \\ebbiog head band. C-10 Ocular Transilluminator. E .S.I.Co. ·c-11 Ophthalmoscope. Loring's, 1 len . "C-12 Oph halmoscope, loring's, lectrical, with mtrror, in ca . •c-13 Ophthalmoscope, Morton 's, lectrical. with mtrror. on battery hand! . · C- 14 Retinoscope ?o.lirror. r lain or conca c. 11 mch . regtlar hand! . ·c-1s Retinoscope Mtrror, r lain or conca e, 1Y2 inchc , folding handle ·c-1 R tino ope firror, Thor ington's, % inch. •C- 17 Retino · ope ftrror, T h or ington's, % inch, with folding cover ·c-1 Retinoscope, lectrical, on battery handle, in case. •C-19 Retinoscope and Ophthalmoscope, electrical, on battery handle, in case. ·c-20 Ashe tos Chimney, with iris dtaphragm. Eyelid Retractors C-30 Desrna rre's, 4 izes. ·c-a Fish er 's, lid hook, double. latest model. C-3 Fi h er 's, fen trated. flexibl for shaping. C-37 Fisher 's, solid, fl xiblc for haping. C- Fish er 's, for lov r ltd. C 0 Ziegler's, one size. P A CE 170 STANDARD SURGICAL INSTRUMENTS ( ,)() . -

Aesculap Spine Miaspas TL
Aesculap Spine Miaspas TL Spinal Microsurgical Endoscopy THORACOSCOPIC LAPAROSCOPIC CONTENTS PAGE INTRODUCTION 3 RONGEURS AND NUCLEUS-GRASPING FORCEPS 5 BONE PUNCHES 6 APPLICATION OF THE SLIDING TUBE 7 DISSECTORS AND EXPLORATION HOOKS 8 SCOOPS 9 CURETTES, OSTEOTOMES AND IMPACTORS 10 LUNG SPATULA AND RIB ELEVATOR 11 SPECULUM, DILATOR ROD, BONE-GRAFT-HOLDER AND BONE GRAFT MEASUREMENT INSTRUMENT 12 DRILL, BURRS AND HAND PIECE 13 TROCARS COMPLETE 14 TROCAR SYSTEM 16 BIPOLAR FORCEPS 17 FURTHER ENDOSCOPIC INSTRUMENTS AND MOTOR LINE EQUIPMENT SEE FOLLOWING LEAFLETS 18 AESCULAP SPINE PRODUCTS 19 2 SPINAL MICROSURGICAL ENDOSCOPY The miaspasTL instrument system was developed in co-operation with Daniel Rosenthal, MD. Praxisklinik Bad Homburg Hessenring 128 A 61348 Bad Homburg Germany to safely perform microsurgical endoscopic surgery on the thoracic and lumbar spine. Microsurgical endoscopy - as the name suggests - is a technique based on two of the most minimally invasive or least traumatic current techniques for spinal surgery. The miaspasTL instruments are designed with the surgeon in mind, to facilitate spinal microsurgical endoscopic procedures. Main advantages of the miaspasTL instrument system: ➠ microsurgical endoscopic technique promotes minimal patient trauma ➠ suitable for both laparoscopic (using gas insufflation) and thoracoscopic (gasless) procedures ➠ well balanced instrument design ensures easy handling ➠ high quality reusable instruments ensure cost savings ➠ instrumentation is easily disassembled for cleaning prior to sterilisation -

United States Patent (19) 11 Patent Number: 4,545,374 Jacobson 45 Date of Patent: Oct
United States Patent (19) 11 Patent Number: 4,545,374 Jacobson 45 Date of Patent: Oct. 8, 1985 54 METHOD AND INSTRUMENTS FOR PERFORMING A PERCUTANEOUS OTHER PUBLICATIONS LUMBAR DISKECTOMY "Nucleography”, May 1952, issue of Journal of Bone (76) Inventor: Robert E. Jacobson, 1295 NW. 14th and Joint Surgery, vol. 34B, No. 2. St., Suite G, Miami, Fla. 33125 "Dr. Parvas Kanbin Develops New Surgical Procedure Aiding Herniated Spinal Disk Sufferers', May 1982, 21) Appl. No.: 414,779 issue of Image, vol. 6, No. 5. 22) Filed: Sep. 3, 1982 "Microlumbar Discectomy', Feb. 1982, issue of Resi dent and Staff Physician. (51) Int. Cl." .............................................. A61B 17/00 52 U.S. C. ................................ 128/303 R; 128/305; "Oh, My Aching Back', p. D1, Saturday, Nov. 7, 1981, 128/312; 128/348.1; 604/164; 604/264 issue of The Miami Herald. 58) Field of Search .............. 128/348.1, 92 E, 92 EB, "Percutaneous Lumbar Discectomy', 1981, Dr. Robert 128/303 R, 753-754, 341-343, 345, 3, 126, E. Jacobson. 305.3, 741, 303.11, 303.13,783-784, 362; Primary Examiner-C. Fred Rosenbaum 604/22, 164-166, 264,904 Assistant Examiner-Gene B. Kartchner 56) References Cited Attorney, Agent, or Firm-Pennie & Edmonds U.S. PATENT DOCUMENTS (57) ABSTRACT 2,991,787 7/1961 Shelden et al. .................. 128/305.3 A method for percutaneously accessing the lumbar 3,308,819 3/1967 Arp ..................................... 604/164 region of the spinal column by laterally inserting a can 3,320,131 5/1967 Smith . nula through the patient's side above the pelvic crest to 3,320,948 5/1967 Martin ..................................