Contents 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Instrument 43-101 Technical Report
ASANKO GOLD MINE – PHASE 1 DEFINITIVE PROJECT PLAN National Instrument 43-101 Technical Report Prepared by DRA Projects (Pty) Limited on behalf of ASANKO GOLD INC. Original Effective Date: December 17, 2014 Amended and Restated Effective January 26, 2015 Qualified Person: G. Bezuidenhout National Diploma (Extractive Metallurgy), FSIAMM Qualified Person: D. Heher B.Sc Eng (Mechanical), PrEng Qualified Person: T. Obiri-Yeboah, B.Sc Eng (Mining) PrEng Qualified Person: J. Stanbury, B Sc Eng (Industrial), Pr Eng Qualified Person: C. Muller B.Sc (Geology), B.Sc Hons (Geology), Pr. Sci. Nat. Qualified Person: D.Morgan M.Sc Eng (Civil), CPEng Asanko Gold Inc Asanko Gold Mine Phase 1 Definitive Project Plan Reference: C8478-TRPT-28 Rev 5 Our Ref: C8478 Page 2 of 581 Date and Signature Page This report titled “Asanko Gold Mine Phase 1 Definitive Project Plan, Ashanti Region, Ghana, National Instrument 43-101 Technical Report” with an effective date of 26 January 2015 was prepared on behalf of Asanko Gold Inc. by Glenn Bezuidenhout, Douglas Heher, Thomas Obiri-Yeboah, Charles Muller, John Stanbury, David Morgan and signed: Date at Gauteng, South Africa on this 26 day of January 2015 (signed) “Glenn Bezuidenhout” G. Bezuidenhout, National Diploma (Extractive Metallurgy), FSIAMM Date at Gauteng, South Africa on this 26 day of January 2015 (signed) “Douglas Heher” D. Heher, B.Sc Eng (Mechanical), PrEng Date at Gauteng, South Africa on this 26 day of January 2015 (signed) “Thomas Obiri-Yeboah” T. Obiri-Yeboah, B.Sc Eng (Mining) PrEng Date at Gauteng, South Africa on this 26 day of January 2015 (signed) “Charles Muller” C. -

Stillwater Mine, 45°23'N, 109°53'W East Boulder Mine, 45°30'N, 109°05'W
STILLWATER MINING COMPANY TECHNICAL REPORT FOR THE MINING OPERATIONS AT STILLWATER MINING COMPANY STILLWATER MINE, 45°23'N, 109°53'W EAST BOULDER MINE, 45°30'N, 109°05'W (BEHRE DOLBEAR PROJECT 11-030) MARCH 2011 PREPARED BY: MR. DAVID M. ABBOTT, JR., CPG DR. RICHARD L. BULLOCK, P.E. MS. BETTY GIBBS MR. RICHARD S. KUNTER BEHRE DOLBEAR & COMPANY, LTD. 999 Eighteenth Street, Suite 1500 Denver, Colorado 80202 (303) 620-0020 A Member of the Behre Dolbear Group Inc. © 2011, Behre Dolbear Group Inc. All Rights Reserved. www.dolbear.com Technical Report for the Mining Operations at Stillwater Mining Company March 2011 TABLE OF CONTENTS 3.0 SUMMARY ..................................................................................................................................... 1 3.1 INTRODUCTION .............................................................................................................. 1 3.2 EXPLORATION ................................................................................................................ 1 3.3 GEOLOGY AND MINERALIZATION ............................................................................ 2 3.4 DRILLING, SAMPLING METHOD, AND ANALYSES ................................................. 3 3.5 RESOURCES AND RESERVES ....................................................................................... 3 3.6 DEVELOPMENT AND OPERATIONS ........................................................................... 5 3.6.1 Mining Operation .................................................................................................. -

Gravity Concentration in Artisanal Gold Mining
minerals Review Gravity Concentration in Artisanal Gold Mining Marcello M. Veiga * and Aaron J. Gunson Norman B. Keevil Institute of Mining Engineering, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; [email protected] * Correspondence: [email protected] Received: 21 September 2020; Accepted: 13 November 2020; Published: 18 November 2020 Abstract: Worldwide there are over 43 million artisanal miners in virtually all developing countries extracting at least 30 different minerals. Gold, due to its increasing value, is the main mineral extracted by at least half of these miners. The large majority use amalgamation either as the final process to extract gold from gravity concentrates or from the whole ore. This latter method has been causing large losses of mercury to the environment and the most relevant world’s mercury pollution. For years, international agencies and researchers have been promoting gravity concentration methods as a way to eventually avoid the use of mercury or to reduce the mass of material to be amalgamated. This article reviews typical gravity concentration methods used by artisanal miners in developing countries, based on numerous field trips of the authors to more than 35 countries where artisanal gold mining is common. Keywords: artisanal mining; gold; gravity concentration 1. Introduction Worldwide, there are more than 43 million micro, small, medium, and large artisanal miners extracting at least 30 different minerals in rural regions of developing countries (IGF, 2017) [1]. Approximately 20 million people in more than 70 countries are directly involved in artisanal gold mining (AGM), with an estimated gold production between 380 and 450 tonnes per annum (tpa) (Seccatore et al., 2014 [2], Thomas et al., 2019 [3], Stocklin-Weinberg et al., 2019 [4], UNEP, 2020 [5]). -

On the Association of Palladium-Bearing Gold, Hematite and Gypsum in an Ouro Preto Nugget
473 The Canadian Mineralogist Vol. 41, pp. 473-478 (2003) ON THE ASSOCIATION OF PALLADIUM-BEARING GOLD, HEMATITE AND GYPSUM IN AN OURO PRETO NUGGET ALEXANDRE RAPHAEL CABRAL§ AND BERND LEHMANN Institut für Mineralogie und Mineralische Rohstoffe, Technische Universität Clausthal, Adolph-Roemer-Str. 2A, D-38678 Clausthal-Zellerfeld, Germany ROGERIO KWITKO-RIBEIRO§ Centro de Desenvolvimento Mineral, Companhia Vale do Rio Doce, Rodovia BR 262/km 296, Caixa Postal 09, 33030-970 Santa Luzia – MG, Brazil RICHARD D. JONES 1636 East Skyline Drive, Tucson, Arizona 85178, U.S.A. ORLANDO G. ROCHA FILHO Mina do Gongo Soco, Companhia Vale do Rio Doce, Fazenda Gongo Soco, Caixa Postal 22, 35970-000 Barão de Cocais – MG, Brazil ABSTRACT An ouro preto (black gold) nugget from Gongo Soco, Minas Gerais, Brazil, has a mineral assemblage of hematite and gypsum hosted by Pd-bearing gold. The hematite inclusion is microfractured and stretched. Scattered on the surface of the gold is a dark- colored material that consists partially of Pd–O with relics of palladium arsenide-antimonides, compositionally close to isomertieite and mertieite-II. The Pd–O coating has considerable amounts of Cu, Fe and Hg, and a variable metal:oxygen ratio, from O-deficient to oxide-like compounds. The existence of a hydrated Pd–O compound is suggested, and its dehydration or deoxygenation at low temperatures may account for the O-deficient Pd-rich species, interpreted as a transient phase toward native palladium. Although gypsum is a common mineral in the oxidized (supergene) zones of gold deposits, the hematite–gypsum- bearing palladian gold nugget was tectonically deformed under brittle conditions and appears to be of low-temperature hydrother- mal origin. -

The Geological Occurrence, Mineralogy, and Processing by Flotation of Platinum Group Minerals (Pgms) in South Africa and Russia
minerals Review The Geological Occurrence, Mineralogy, and Processing by Flotation of Platinum Group Minerals (PGMs) in South Africa and Russia Cyril O’Connor 1,* and Tatiana Alexandrova 2 1 Department of Chemical Engineering, Centre for Minerals Research, University of Cape Town, Cape Town 7701, South Africa 2 Department of Minerals Processing, St Petersburg Mining University, St Petersburg 199106, Russia; [email protected] * Correspondence: [email protected] Abstract: Russia and South Africa are the world’s leading producers of platinum group elements (PGEs). This places them in a unique position regarding the supply of these two key industrial commodities. The purpose of this paper is to provide a comparative high-level overview of aspects of the geological occurrence, mineralogy, and processing by flotation of the platinum group minerals (PGMs) found in each country. A summary of some of the major challenges faced in each country in terms of the concentration of the ores by flotation is presented alongside the opportunities that exist to increase the production of the respective metals. These include the more efficient recovery of minerals such as arsenides and tellurides, the management of siliceous gangue and chromite in the processing of these ores, and, especially in Russia, the development of novel processing routes to recover PGEs from relatively low grade ores occurring in dunites, black shale ores and in vanadium-iron-titanium-sulphide oxide formations. Keywords: Russia; South Africa; PGMs; geology; mineralogy; flotation Citation: O’Connor, C.; Alexandrova, T. The Geological Occurrence, Mineralogy, and Processing by Flotation of Platinum Group Minerals (PGMs) in South 1. Introduction Africa and Russia. -

NI 43-101 Report Template
Report to: Technical Report on the Magino Property, Wawa, Ontario Document No. 1295890100-REP-R0001-02 1295890100-REP-R0001-02 Report to: TECHNICAL REPORT ON THE MAGINO PROPERTY,WAWA,ONTARIO EFFECTIVE DATE:OCTOBER 4, 2012 Prepared by Patrick Huxtable, MAIG (RPGeo) Todd McCracken, P.Geo. Todd Kanhai, P.Eng. PT/JW/jc 1295890100-REP-R0001-02 Report to: TECHNICAL REPORT ON THE MAGINO PROPERTY,WAWA,ONTARIO EFFECTIVE DATE:OCTOBER 4, 2012 “Original document signed by Prepared by Patrick Huxtable, MAIG (RPGeo)” Date October 4, 2012 Patrick Huxtable, MAIG (RPGeo) “Original document signed by Prepared by Todd McCracken, P.Geo.” Date October 4, 2012 Todd McCracken, P.Geo. “Original document signed by Prepared by Todd Kanhai, P.Eng.” Date October 4, 2012 Todd Kanhai, P.Eng. “Original document signed by Reviewed by Jeff Wilson, Ph.D., P.Geo.” Date October 4, 2012 Jeff Wilson, Ph.D., P.Geo. “Original document signed by Authorized by Jeff Wilson, Ph.D., P.Geo.” Date October 4, 2012 Jeff Wilson, Ph.D., P.Geo. PT/JW/jc Suite 900, 330 Bay Street, Toronto, Ontario M5H 2S8 Phone: 416-368-9080 Fax: 416-368-1963 1295890100-REP-R0001-02 REVISION HISTORY REV. PREPARED BY REVIEWED BY APPROVED BY NO ISSUE DATE AND DATE AND DATE AND DATE DESCRIPTION OF REVISION 00 2012/09/20 Patrick Huxtable Jeff Wilson Jeff Wilson Draft to Client for review. Todd McCracken 01 2012/09/27 Patrick Huxtable Jeff Wilson Jeff Wilson Draft to Client for review. Todd McCracken 02 2012/10/04 Patrick Huxtable Jeff Wilson Jeff Wilson Final to Client. -

Scavenging Flotation Tailings Using a Continuous Centrifugal Gravity Concentrator
Scavenging Flotation Tailings using a Continuous Centrifugal Gravity Concentrator by Hassan Ghaffari B.A.Sc. & M.A.Sc. Department of Mining Engineering, Technical Faculty Tehran University, Iran, 1990 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Applied Science in THE FACULTY OF GRADUATE STUDIES DEPARTMENT OF MINING ENGINEERIG THE UNIVERSITY OF BRITISH COLUMBIA We accepted this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA August 2004 © Hassan Ghaffari, 2004 THE UNIVERSITY OF BRITISH COLUMBIA FACULTY OF GRADUATE STUDIES Library Authorization In presenting this thesis in partial fulfillment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. H ASS A A/ GrMFFAR I 31 ,o%2t>4 Name of Author (please print) Date (dd/mm/yyyy) Title of Thesis: Degree: /l/l-A S C Year: Department of The University of British Columbiumbia ^ u c/ Vancouver, BC Canada grad.ubc.ca/forms/?formlD=THS page 1 of 1 last updated: 31-Aug-04 11 Summary A study was conducted to evaluate the Knelson Continuous Variable Discharge (CVD) concentrator as a scavenger for coarse middling particles from flotation tailings. The goal was to recover a product of suitable grade for recycling to the grinding circuit to improve liberation and aid subsequent recovery in flotation. -

Knelson Concentrator
UPGRADING OF GOLD GRAVITY CONCENTRATES A STUDY OF THE KNELSON CONCENTRATOR Liming Huang A thesis subrnitted to the Faculty of Graduate Studies and Research in partial fuifiiiment of the requirements for the Degree of Philosophy Department of Mining and Metaliurgical Engineering, McGill University Montreal, Canada National Library Bibliothèque nationale 1*1 of Canada du Canada Acquisitions and Acquisitions et Bibliographie Services services bibliographiques 395 Wellington Street 395. nie Wellington ûîtawaON K1AON4 OttawaON K1AOW Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence dowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sell reproduire, prêter, distribuer ou copies of ths thesis in microfom, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/£ïlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantid extracts from it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. ABSTRACT In recent years. the Knelson Concentrator has become the predominant unit used for primary goid recovery by gravity . However. its application potential as a cleaner in the final gravity concentration stage and its separation mechanisms have not been well studied. In most Canadian gold mills. rougher gravity concentrates produced by the Knelson (operating at 60 gravity acceleration or 'g') are further upgraded with shaking tables. -

Flotation and Gravity Separation
'r 1 TECHNICAL, REPORT December 1,1994 through February 28, 1995 Project Title: A FINE COAL CIRCUITRY STUDY USING COLUMN FLOTATION AND GRAVITY SEPARATION DOE Cooperative Agreement Number: DE-FC22-92PC92521 (Year 3) ICCI Project Number: 94-l/l.lA-lP Principal Investigator: Ricky Q. Honaker Department of Mining Engineering Southern Illinois University Other Investigators: Stephen Reed Kerr-McGee Coal Corporation Project Manager: Ken Ho, Illinois Clean Coal Institute ABSTRACT Column flotation provides excellent recovery of ultrafine coal while producing low ash content concentrates. However, column flotation is not efficient for treating fine coal containing sigtllficant amounts of mixed-phase particles. Fortunately, enhanced gravity separation has proved to have the ability to treat the mixed-phased particles more effectively. A disadvantage of gravity separation is that ultrafine clay particles are not easily rejected. Thus, a combination of these two technologies may provide a circuit that maximizes both the ash and sulfur rejection that can be achieved by physical coal cleaning while maintaining a high energy recovery. This project is studying the potential of using different combinations of gravity separators, i.e., a Floatex hydrosizer and a Falcon Concentrator, and a proven flotation column, which will be selected based on previous studies by the principle investigator. During this reporting period, an in-plant Box-Behnken test program of the Floatex hydrosizer has been conducted at Kerr-McGee's Galatia preparation plant. The results have shown that the Floatex hydrosizer can be successfblly used to reject most of coarser (+lo0 mesh) pyrite and mineral matter in the coal stream to the plant. -

British Geological Survey
BRITISH GEOLOGICAL SURVEY Mineralogy and Petrology Short Report Series Short Report No. MPSR/99/24 RECOVERING THE LOST GOLD OF THE DEVELOPING WORLD: BIBLIOGRAPHIC DATABASE E J Evans & C J Mitchell Date 31/03/99 Classification Open Geographical Index World-wide Subject index Gold, mercury, mineral processing, gravity, sluice, Bibliographic reference E J Evans & C J Mitchell Recovering the lost gold of the developing world: bibliographic database Mineralogy and Petrology Group Short Report MPSR/99/24 ©NERC 1999 British Geological Survey, Keyworth, Nottingham. NG12 5GG. UK. MPSR/99/24 31103/99 BRITISH GEOLOGICAL SURVEY Mineralogy and Petrology Group Short Report No. MPSR/99/24 Recovering the lost gold of the developing world: bibliographic database E J Evans & C J Mitchell INTRODUCTION This report contains a library of 181 references, including abstracts, prepared for Project R 7120 "Recovering the lost gold of the developing world" funded by the UK' s Department for International Development (DFID) under the Knowledge and Research (KAR) programme. As part of an initial desk study, a literature review of gold processing methods used by small-scale miners was carried out using the following sources; the lSI Science Citation Index accessed via Bath Information and Data Services (BIDS), a licensed GEOREF CD-ROM database held at the BGS's Library in Keyworth and IMMage a CD-ROM database produced by the Institution of Mining and Metallurgy held by the Minerals group ofBGS. Information on the search terms used is available from the author. Results of this literature review were compiled in digital form as an Endnote® bibliographic library containing 181 individual references. -

Towards the Recovery of Rare Earth Elements from End-Of-Life Products: Hydrometallurgical Routes and Mathematical Modelling of Extraction Systems
Chemical Engineering Department PhD Program: Chemical Processes Engineering TOWARDS THE RECOVERY OF RARE EARTH ELEMENTS FROM END-OF-LIFE PRODUCTS: HYDROMETALLURGICAL ROUTES AND MATHEMATICAL MODELLING OF EXTRACTION SYSTEMS Author: Eleonora Obón Estrada Director: Dr. Ana Maria Sastre Requena Codiretor: Dr. Agustín Fortuny Sanromà Escola Tècnica Superior d’Enginyeria Industrial de Barcelona (ETSEIB) Universitat Politècnica de Catalunya, Barcelona, July 2019 Thesis presented to obtain the qualification of Doctor awarded by the Universitat Politècnica de Catalunya II Abstract The rare earth elements (REEs) are essential ingredients for the development of modern industry and the transition to a more sustainable economy model. The unique physicochemical features of these elements, such as their magnetism and optical properties, are greatly expanding their application. They have become key elements for the manufacture of many ordinary consumer goods like hybrid cars, fluorescent lamps or electronic devices like mobile phones or tablets. The growing popularity of the rare earth elements derivatives is leading to an increase in the global demand and the price of these elements. Unfortunately, the current availability of these resources is limited due to three main factors: their heterogeneous geological location, their low concentration in the ores, and the environmental issues related with their mining. All these disadvantages concerning the supply of the rare earth elements have led to the study of new techniques to obtain them, such as the recycling of end- of-life products. Recycling of REEs arises as a new secondary source of supply of REEs, especially in Europe where large amounts of technological waste are generated every year. Currently, the recycling of rare earth elements represents less than 1% of the global supply. -
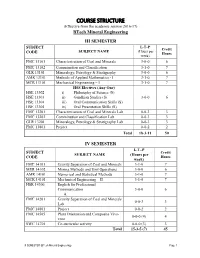
COURSE STRUCTURE (Effective from the Academic Session 2016-17) Btech Mineral Engineering
COURSE STRUCTURE (Effective from the academic session 2016-17) BTech Mineral Engineering III SEMESTER SUBJECT L-T–P Credit SUBJECT NAME (Hours per CODE Hours week) FMC 13101 Characterisation of Coal and Minerals 3-0-0 6 FMC 13102 Comminution and Classification 3-1-0 7 GLR 13101 Mineralogy, Petrology & Stratigraphy 3-0-0 6 AMR 13101 Methods of Applied Mathematics - I 3-1-0 7 MCR 13101 Mechanical Engineering – I 3-1-0 7 HSS Electives (Any One) HSE 13302 i) Philosophy of Science (S) HSE 13303 ii) Gandhian Studies (S) 3-0-0 6 HSE 13304 iii) Oral Communication Skills (S) HSE 13305 iv) Oral Presentation Skills (S) FMC 13201 Characterisation of Coal and Minerals Lab 0-0-3 3 FMC 13202 Comminution and Classification Lab 0-0-3 3 GLR 13201 Mineralogy, Petrology & Stratigraphy Lab 0-0-3 3 FMC 13801 Project 0-0-2 2 Total 18-3-11 50 IV SEMESTER L-T–P SUBJECT Credit SUBJECT NAME (Hours per CODE Hours week) FMC 14101 Gravity Separation of Coal and Minerals 3-1-0 7 MER 14102 Mining Methods and Unit Operations 3-0-0 6 AMR 14101 Numerical and Statistical Methods 3-1-0 7 MCR 14101 Mechanical Engineering – II 3-1-0 7 HSR 14306 English for Professional Communication 3-0-0 6 A. FMC 14201 Gravity Separation of Coal and Minerals 0-0-3 3 Lab FMC 14801 Project 0-0-2 2 FMC 14505 Plant Orientation and Composite Viva- 0-0-0 (4) 4 voce SWC 14701 Co-curricular activity 0-0-0 (3) 3 Total 15-3-5 (7) 45 III SEMESTER (BTech Mineral Engineering) Page 1 V SEMESTER L-T–P SUBJECT SUBJECT NAME (Hours per Credit Hours CODE week) BTech MINERAL ENGINEERING FMC 15101 Magnetic, Electrical