Auxiliary Label List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photoaging & Skin Damage
Use_for_Revised_OFC_Only_2006_PhotoagingSkinDamage 5/21/13 9:11 AM Page 2 PEORIA (309) 674-7546 MORTON (309) 263-7546 GALESBURG (309) 344-5777 PERU (815) 224-7400 NORMAL (309) 268-9980 CLINTON, IA (563) 242-3571 DAVENPORT, IA (563) 344-7546 SoderstromSkinInstitute.comsoderstromskininstitute.com FROMFrom YOUR Your DERMATOLOGISTDermatologist [email protected]@skinnews.com PHOTOAGING & SKIN DAMAGE Before You Worship The Sun Who’s At Risk? Today, many researchers and dermatologists Skin types that burn easily and tan rarely are believe that wrinkling and aging changes of the skin much more susceptible to the ravages of the sun on the are much more related to sun damage than to age! skin than are those that tan easily, rather than burn. Many of the signs of skin damage from the sun are Light complected, blue-eyed, red-haired people such as pictured on these pages. The decrease in the ozone Swedish, Irish, and English, are usually more suscep- layer, increasing the sun’s intensity, and the increasing tible to photo damage, and their skin shows the signs sun exposure among our population – through work, of photo damage earlier in life and in a more pro- sports, sunbathing and tanning parlors – have taken a nounced manner. Dark complexions give more protec- tremendous toll on our skin. Sun damage to the skin tion from light and the sun. ranks with other serious health dangers of smoking, alcohol, and increased cholesterol, and is being seen in younger and younger people. NO TAN IS A SAFE TAN! Table of Contents Sun Damage .............................................Pg. 1 Skin Cancer..........................................Pgs. 2-3 Mohs Micrographic Surgery ......................Pg. -

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

Late Stent Thrombosis After Paclitaxel-Eluting Stent Placement in a Patient with Essential Thrombocytosis
558 Türk Kardiyol Dern Arş - Arch Turk Soc Cardiol 2010;38(8):558-560 Late stent thrombosis after paclitaxel-eluting stent placement in a patient with essential thrombocytosis Esansiyel trombositozlu bir hastada paklitaksel salınımlı stent yerleştirme sonrası gelişen geç stent trombozu Telat Keleş, M.D., Nihal Akar Bayram, M.D., Tahir Durmaz, M.D., Engin Bozkurt, M.D. Department of Cardiology, Ankara Atatürk Education and Research Hospital, Ankara We report on a case of late stent thrombosis after drug- Bu yazıda, esansiyel trombositozlu bir hastada ilaç sa- eluting stent placement in a patient with essential throm- lınımlı stent yerleştirme sonrası gelişen geç stent trom- bocytosis. A 51-year-old male patient with a three-month bozu sunuldu. Üç ay önce sol ön inen artere paklitak- history of paclitaxel-eluting stent placement to the left sel salınımlı stent yerleştirilen 51 yaşındaki erkek hasta anterior descending artery presented with a complaint şiddetli retrosternal göğüs ağrısı yakınmasıyla başvur- of severe retrosternal chest pain. A high platelet count du. İki ay öncesinde hastanın trombosit sayımı yüksek (1,063,000/mm3) was detected two months prior to (1063000/mm3) bulunmuş ve durumu esansiyel trombo- presentation, which was interpreted as essential throm- sitoz olarak yorumlanmıştı. Hasta standart ikili antitrom- bocytosis. He was on standard dual antiplatelet therapy bosit tedavi (aspirin ve klopidogrel) görmekteydi. Elekt- (aspirin and clopidogrel). The electrocardiogram showed rokardiyografide V1-V6 derivasyonlarında ST-segment ST-segment elevation in leads V1-V6. Emergent coro- yükselmesi izlendi. Acil koroner anjiyografide paklitaksel nary angiography revealed thrombotic total occlusion salınımlı stent yerinde trombotik tam tıkanıklık gözlendi. at the location of the paclitaxel-eluting stent. -

May 2020 for Health Professionals Who Care for Cancer Patients
Systemic Therapy Update Volume 23 Issue 5 May 2020 For Health Professionals Who Care for Cancer Patients Inside This Issue: Editor’s Choice Benefit Drug List New Programs: Neoadjuvant Carboplatin for Triple-Negative New: BRLACTWAC, UGUPAPA, Pegaspargase (LKNOS, Breast Cancer (BRLACTWAC) | Apalutamide for Non- LYNOS), Bevacizumab (Pediatric), IGEV (Pediatric) | Metastatic Castration-Resistant Prostate Cancer (UGUPAPA) Deleted: GIGAVEOCAP, GIGAVEOF, SMAJIFN Drug Update NEW Protocols, PPPOs and Patient Handouts Pharmacare Update – Anticoagulants | Patient Assistance BR: BRLACTWAC | GU: UGUPAPA Programs REVISED Protocols, PPPOs and Patient Handouts Drug Shortages BR: BRAJAC, BRAJACT, BRAJACTG, BRAJACTT, BRAJACTTG, New: Alemtuzumab, Anagrelide | Updated: Hydroxyurea BRAJACTW, BRAJDAC, BRAJFEC, BRAJFECD, BRAJFECDT, Cancer Drug Manual© BRAVAC, BRLAACD, BRLAACDT, BRLATACG, BRLATWAC | New: Abemaciclib, Lurbinectedin | Revised: Apalutamide, GI: GIPGEMABR | GU: UGUPABI, UGUPENZ | LK: ULKBLIN, Durvalumab, Panitumumab, Pembrolizumab ULKMDSA | SA: SAIME | SC: SCDRUGRX | SM: USMAVDAB, ST Update Editorial Board USMAVDT, USMAVTRA, USMAVVC, USMAVVEM Membership Update Resources and Contact Information Editor’s Choice New Programs Effective 01 May 2020, the BC Cancer Provincial Systemic Therapy Program has approved the following new treatment programs. The full details of these programs can be found on the BC Cancer website in the Chemotherapy Protocols section. Breast Neoadjuvant Carboplatin for Locally Advanced, Triple-Negative Breast Cancer (BRLACTWAC) — The Provincial Systemic Therapy Program has approved the addition of carboplatin to the standard taxane- anthracycline neoadjuvant chemotherapy regimens, for patients with stage II and higher triple-negative breast cancer. This regimen consists of carboplatin (given every 3 weeks) in combination with weekly paclitaxel, followed by doxorubicin-cyclophosphamide (AC given every 2 or 3 weeks). Filgrastim support is available for the dose-dense AC option. -
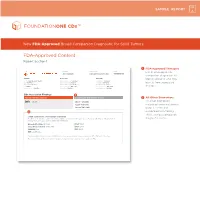
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y
Published OnlineFirst November 6, 2015; DOI: 10.1158/1078-0432.CCR-15-1588 Clinical Trial Brief Report Clinical Cancer Research An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y. Sarin, Anthony E. Oro, and Anne Lynn S. Chang Abstract Purpose: To assess the tumor response to the smoothened sive disease with sonidegib. Three patients experienced stable (SMO) inhibitor, sonidegib (LDE225), in patients with an disease and discontinued sonidegib either due to adverse events advanced basal cell carcinoma (BCC) resistant to treatment with (n ¼ 1) or due to election for surgery (n ¼ 2). The response of one vismodegib (GDC0449). patient was not evaluable. SMO mutations with in vitro data Experimental Design: Nine patients with an advanced suggesting resistance to Hh pathway inhibition were identified BCC that was previously resistant to treatment with vismode- in 5 patients, and none of these patients experienced responses gib were given sonidegib in this investigational, open- while on sonidegib. label study. Tumor response was determined using the Conclusion: Patients with advanced BCCs that were response evaluation criteria in solid tumors. SMO mutations previously resistant to treatment with vismodegib similarly were identified using biopsy samples from the target BCC demonstrated treatment resistance with sonidegib. Patients location. who have developed treatment resistance to an SMO inhibitor Results: The median duration of treatment with sonidegib was may continue to experience tumor progression in response to 6 weeks (range, 3–58 weeks). Five patients experienced progres- other SMO inhibitors. Clin Cancer Res; 1–5. Ó2015 AACR. Introduction Sonidegib (LDE225) is a new SMO inhibitor approved in 2015 by the FDA for locally advanced BCCs. -

Resistance to Antifolates
Oncogene (2003) 22, 7431–7457 & 2003 Nature Publishing Group All rights reserved 0950-9232/03 $25.00 www.nature.com/onc Resistance to antifolates Rongbao Zhao1 and I David Goldman*,1 1Departments of Medicine and Molecular, Pharmacology, Albert Einstein College of Medicine, Bronx, New York, USA The antifolates were the first class of antimetabolites to the kinetics of the interaction between MTX and DHFR enter the clinics more than 50 years ago. Over the was fully understood, and not until the late 1970s and following decades, a full understanding of their mechan- early 1980s when polyglutamate derivatives of MTX were isms of action and chemotherapeutic potential evolved detected and their pharmacologic importance clarified. along with the mechanisms by which cells develop Likewise, an understanding of tumor cell resistance to resistance to these drugs. These principals served as a antifolates evolved slowly, often paralleling the emergence basis for the subsequent exploration and understanding of of new molecular concepts. As the mechanisms of the mechanisms of resistance to a variety of diverse resistance to antifolates were characterized, this provided antineoplastics with different cellular targets. This section insights and principles that were broadly applicable to describes the bases for intrinsic and acquired antifolate other antineoplastics. Ultimately, this knowledge led to the resistance within the context of the current understanding development of a new generation of antifolates, in the late of the mechanisms of actions and cytotoxic determinants 1980s and 1990s, which are potent direct inhibitors of of these agents. This encompasses impaired drug transport tetrahydrofolate (THF)-cofactor-dependent enzymes. Sev- into cells, augmented drug export, impaired activation of eral of these drugs are now in clinical trials, and the antifolates through polyglutamylation, augmented hydro- activity of one, pemetrexed, has been confirmed in a large lysis of antifolate polyglutamates, increased expression Phase III trial (Vogelzang et al., 2003). -

(ATRA) with Arsenic Trioxide (ATO) Induction Therapy
NCCP Chemotherapy Regimen Tretinoin (ATRA) with Arsenic Trioxide (ATO) Induction Therapy INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10 Code Status Treatment of patients with newly diagnosed low to intermediate C92 00356a Hospital risk Acute Promyelocytic Leukaemia (APL) *If the reimbursement status is not defined i, the indication has yet to be assessed through the formal HSE reimbursement process. TREATMENT: The starting dose of the drugs detailed below may be adjusted downward by the prescribing clinician, using their independent medical judgement, to consider each patients individual clinical circumstances. The treatment is administered until haematological complete remission (CR) or for a maximum of 60 days. Patients who achieve haematological CR progress to Consolidation Therapy with all trans retinoic acid (ATRA) and Arsenic Trioxide (Reference NCCP Regimen 00357). CR is defined as where the bone marrow is regenerating normal haematopoietic cells and contains <5% blast cells by morphology in an aspirate sample with at least 200 nucleated cells. Day Drug Dose Route Cycle 1 until complete Tretinoin 45mg/m2 in aPO n/a Continuous until remission (ATRA) divided doses Complete Remission (CR) is achieved or up to a maximum of 60 days 1-5 inclusive Arsenic 0.3mg/kg IV 250ml of 0.9% NaCl over Week 1 only trioxide infusion 2 hoursb Twice weekly Arsenic 0.25mg/kg IV 250ml of 0.9% NaCl over Week 2 to 8 trioxide infusion 2 hoursb 1 until end of Prednisolone 0.5mg/kg/day PO induction a Tretinoin (ATRA) is available as 10mg capsules. Round dose to nearest 10mg. The capsules should be swallowed whole with water. -

Imatinib (Gleevec™)
Biologicals What Are They? When Did All of this Happen? There are Clear Benefits. Are there also Risks? Brian J Ward Research Institute of the McGill University Health Centre Global Health, Immunity & Infectious Diseases Grand Rounds – March 2016 Biologicals Biological therapy involves the use of living organisms, substances derived from living organisms, or laboratory-produced versions of such substances to treat disease. National Cancer Institute (USA) What Effects Do Steroids Have on Immune Responses? This is your immune system on high dose steroids projects.accessatlanta.com • Suppress innate and adaptive responses • Shut down inflammatory responses in progress • Effects on neutrophils, macrophages & lymphocytes • Few problems because use typically short-term Virtually Every Cell and Pathway in Immune System ‘Target-able’ (Influenza Vaccination) Reed SG et al. Nature Medicine 2013 Nakaya HI et al. Nature Immunology 2011 Landscape - 2013 Antisense (30) Cell therapy (69) Gene Therapy (46) Monoclonal Antibodies (308) Recombinant Proteins (93) Vaccines (250) Other (81) http://www.phrma.org/sites/default/files/pdf/biologicsoverview2013.pdf Therapeutic Category Drugs versus Biologics Patented Ibuprofen (Advil™) Generic Ibuprofen BioSimilars/BioSuperiors ? www.drugbank.ca Patented Etanercept (Enbrel™) BioSimilar Etanercept Etacept™ (India) Biologics in Cancer Therapy Therapeutic Categories • Hormonal Therapy • Monoclonal antibodies • Cytokine therapy • Classical vaccine strategies • Adoptive T-cell or dendritic cells transfer • Oncolytic -

Acitretin; Adapalene; Alitretinoin; Bexarotene; Isotretinoin
8 February 2018 EMA/254364/2018 Pharmacovigilance Risk Assessment Committee (PRAC) Assessment report Referral under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data Retinoids containing medicinal products INN: Acitretin, Adapalene, Alitretinoin, Bexarotene, Isotretinoin, Tretinoin, Tazarotene Procedure number: EMEA/H/A-31/1446 Panretin EMEA/H/A-31/1446/C/000279/0037 Targretin EMEA/H/A-31/1446/C/000326/0043 Note: Assessment report as adopted by the PRAC and considered by the CHMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ......................................................................................... 2 1. Information on the procedure ................................................................. 3 2. Scientific discussion ................................................................................ 3 2.1. Introduction......................................................................................................... 3 2.2. Clinical aspects .................................................................................................... 5 2.3. Data on efficacy ..................................................................................................