In Vitro Shoot Regeneration from Leaves of Hypericum Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Preliminary Survey of Plant Distribution in Ohio.* John H
A PRELIMINARY SURVEY OF PLANT DISTRIBUTION IN OHIO.* JOHN H. SCHAFFNER. The following data are presented as a preliminary basis for field work in determining the natural plant areas of Ohio. It is hoped that the botanists of the State will begin active study of local conditions with a view to determine natural or transition boundaries as well as cataloging local associations. The distri- bution lists are based on herbarium material and more than 15 years of sporadic botanizing in the state. Of course, distribution at present indicates to a considerable extent merely the distri- bution of enthusiastic botanists and their favorite collecting grounds. Nevertheless, enough has been done to indicate in a rough way the general character of our plant geography. The kind of data most important in indicating characteristic areas are as follows:— 1. Meteorological data. 2. Geology, including the nature of the surface rock and soil. 3. Physiography and topography. 4. The actual distribution of characteristic species of plants and to some extent of animals. In Ohio, the following important maps may be studied in this connection:— Meteorology. By Otto E. Jennings in Ohio Naturalist 3: 339-345, 403-409, 1903. Maps I-XII. By J. Warren Smith in Bull. Ohio Agr. Exp. Station No. 235, 1912. Figs. 3-14. Geology. By J. A. Bownocker, A Geological Map of Ohio. 1909. Topography. The maps of the topographic survey, not yet completed. Various geological reports. The eastern half of Ohio is a part of the Alleghany Plateau. The western half belongs to the great interior plain. In Ohio, the Alleghany Plateau consists of a northern glaciated region and a southern non-glaciated region. -

Download Authenticated
Ohio Administrative Code Rule 1501:18-1-03 Endangered and threatened species. Effective: January 30, 2021 (A) The following species of plants are designated as endangered in Ohio. (1) Acer pensylvanicum L., Striped maple. (2) Aconitum noveboracense A. Gray, Northern monkshood. (3) Aconitum uncinatum L., Southern monkshood. (4) Adlumia fungosa (Ait.) Greene, Allegheny-vine. (5) Agalinis auriculata (Michx.) Blake, Ear-leaved-foxglove. (6) Agalinis purpurea (L.) Pennell var. parviflora (Benth.) Boivin, Small purple-foxglove. (7) Agalinis skinneriana (Wood) Britt., Skinner's-foxglove. (8) Ageratina aromatica (L.) Spach, Small white snakeroot. (9) Agrostis elliottiana Schultes, Elliott's bent grass. (10) Amelanchier humilis Wiegand, Low serviceberry. (11) Amelanchier interior E.L. Nielsen, Inland serviceberry. (12) Amphidium mougeotii (Bruch, Schimper and W. Gmbel) Schimper, Mougeot's ice moss. (13) Andropogon glomeratus (Walter) Britton, Bushy broom-sedge. Page 1 (14) Androsace occidentalis Pursh, Western rock-jasmine. (15) Anomobryum filiforme (Dicks.) Solms, Common silver moss. (16) Anomodon viticulosus (Hedw.) Hook. and Taylor, Long tail moss. (17) Arabidopsis lyrata (L.) OKane and Al-Shehbaz, Lyre-leaved rock cress. (18) Arabis patens Sullivant, Spreading rock cress. (19) Arctostaphylos uva-ursi (L.) Spreng., Bearberry. (20) Aralia hispida Vent., Bristly sarsaparilla. (21) Arethusa bulbosa L., Dragon's-mouth. (22) Aristida basiramea Engelm. ex Vasey, Forked three-awn grass. (23) Aristida necopina Shinners, False arrow-feather. (24) Aronia arbutifolia (L.) Pers., Red chokeberry. (25) Asplenium bradleyi D.C. Eaton, Bradley's spleenwort. (26) Asplenium resiliens Kunze, Black-stemmed spleenwort. (27) Astragalus neglectus (T. and G.) Sheld., Cooper's milk-vetch. (28) Baptisia australis (L.) R. Br., Blue false indigo. (29) Barbula indica (Hooker) Sprengel in E.G. -

This Week's Sale Plants
THIS WEEK’S SALE PLANTS (conifers, trees, shrubs, perennials, tropical, tenders, tomatoes, pepper) Botanical Name Common Name CONIFERS Cephalotaxus harringtonia 'Duke Gardens' Japanese Plum Yew Cephalotaxus harringtonia 'Prostrata' Japanese Plum Yew Chamaecyparis obtusa 'Nana Gracilis' Dwarf Hinoki Cypress Cupressus arizonica 'Carolina Sapphire' Arizona Cypress Juniperus conferta 'Blue Pacific' Shore Juniper Juniperus horizontalis 'Wiltonii' Blue Rug Juniper Juniperus virginiana Eastern Red Cedar Taxodium distichum 'Emerald Shadow' Bald Cypress Thuja 'Green Giant' Giant Arborvitae TREES Aesculus ×neglecta 'Erythroblastos' Hybrid Buckeye Aesculus hippocastanum 'Digitata' Horsechestnut Asimina triloba 'Levfiv' Susquehanna™ Pawpaw Asimina triloba 'Wansevwan' Shenandoah™ Pawpaw Asimina triloba Pawpaw Carpinus caroliniana 'J.N. Upright' Firespire™ Musclewood Cercidiphyllum japonicum 'Rotfuchs' Red Fox Katsura Tree Cercidiphyllum japonicum Katsura Tree Davidia involucrata 'Sonoma' Dove Tree Fagus grandifolia American Beech Ginkgo biloba 'Saratoga' Ginkgo Ostrya virginiana Hop Hornbeam Quercus alba White Oak Quercus coccinea Scarlet Oak Quercus phellos Willow Oak SHRUBS Abelia ×grandiflora 'Margarita' Glossy Abelia Abelia ×grandiflora 'Rose Creek' Glossy Abelia Aesculus parviflora var. serotina 'Rogers' Bottlebrush Buckeye Aronia arbutifolia 'Brilliantissima' Chokeberry Aronia melanocarpa 'UCONNAM165' Low Scape® Mound Chokeberry Aucuba japonica 'Golden King' Japanese Aucuba Aucuba japonica 'Marmorata' Japanese Aucuba Berberis ×gladwynensis 'William -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -
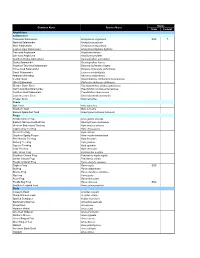
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -
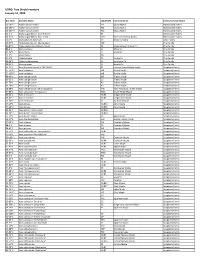
UDBG: Tree Shrub Inventory January 10, 2020
UDBG: Tree Shrub Inventory January 10, 2020 Acc. Num Scientific Name LOCATION Common Name Family Common Name 15-167*1 Abelia 'Canyon Creek' TE2 Glossy Abelia Honeysuckle Family 15-167*2 Abelia 'Canyon Creek' TE2 Glossy Abelia Honeysuckle Family 15-167*3 Abelia 'Canyon Creek' TE2 Glossy Abelia Honeysuckle Family 02-2*1 Abelia x grandiflora 'Little Richard' F5 Honeysuckle Family 18-87*1 Abelia x grandiflora 'Rose Creek' TREE Rose Creek Glossy Abelia Honeysuckle Family 70-1*1 Abeliophyllum distichum C3 White Forsythia Olive Family 15-57*1 Abies balsamea var. phanerolepis NBF Pine Family 90-47*1 Abies cephalonica 'Meyer's Dwarf' C2 Meyer's Dwarf Grecian Fir Pine Family 89-4*1 Abies concolor C1 White Fir Pine Family 01-74*1 Abies firma C1 Momi Fir Pine Family 12-9*7 Abies fraseri PILL Pine Family 71-1*2 Abies koreana C1 Korean Fir Pine Family 95-26*1 Abies nordmanniana C1 Nordmann Fir Pine Family 96-21*1 Abies pinsapo C1 Spanish Fir Pine Family 14-72*1 Acer [Crimson Sunset] = 'JFS-KW202' FE Crimson Sunset hybrid maple Soapberry Family 17-100*1 Acer barbatum WR Florida Maple Soapberry Family 17-100*2 Acer barbatum WR Florida Maple Soapberry Family 88-40*1 Acer buergerianum W2 Trident Maple Soapberry Family 91-32*1 Acer buergerianum C3 Trident Maple Soapberry Family 92-78*1 Acer buergerianum A2 Trident Maple Soapberry Family 92-78*2 Acer buergerianum A2 Trident Maple Soapberry Family 18-28*1 Acer buergerianum 'Mino Yatsubusa' FH5 Mino Yatsubusa Trident Maple Soapberry Family 94-95*1 Acer campsetre 'Compactum' WV1 Dwarf Hedge Maple Soapberry Family -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al. -

Alvaro Cortes Cabrera, Jose M. Prieto, Application of Artificial Neural
Subjek : Minyak Atsiri Tahun 2004-2008 (478 judul) Alvaro Cortes Cabrera, Jose M. Prieto, Application of artificial neural networks to the prediction of the antioxidant activity of essential oils in two experimental in vitro models, Food Chemistry, Volume 118, Issue 1, 1 January 2010, Pages 141-146, ISSN 0308-8146, DOI: 10.1016/j.foodchem.2009.04.070. (http://www.sciencedirect.com/science/article/B6T6R-4W6XYK9- 1/2/708e866c3d7f370d81917ed145af525a) Abstract: Introduction The antioxidant properties of essential oils (EOs) have been on the centre of intensive research for their potential use as preservatives or nutraceuticals by the food industry. The enormous amount of information already generated on this subject provides a rich field for data-miners as it is conceivable, with suitable computational techniques, to predict the antioxidant capacity of any essential oil just by knowing its particular chemical composition. To accomplish this task we here report on the design, training and validation of an Artificial Neural Network (ANN) able to predict the antioxidant activity of EOs of known chemical composition.Methods A multilayer ANN with 30 input neurons, 42 in hidden layers (20 --> 15 --> 7) and one neuron in the output layer was developed and run using Fast Artificial Neural Network software. The chemical composition of 32 EOs and their antioxidant activity in the DPPH and linoleic acid models were extracted from the scientific literature and used as input values. From the initial set of around 80 compounds present in these EOs, only 30 compounds with relevant antioxidant capacity were selected to avoid excessive complexity of the neural network and minimise the associated structural problems.Results and discussion The ANN could predict the antioxidant capacities of essential oils of known chemical composition in both the DPPH and linoleic acid assays with an average error of only 3.16% and 1.46%, respectively. -

Proceedings of the Indiana Academy of Science
List of Extirpated, Endangered, Threatened and Rare Vascular Plants in Indiana: An Update James R. Aldrich, John A. Bacone and Michael A. Homoya Indiana Department of Natural Resources Indianapolis, Indiana 46204 Introduction The status of Indiana's rarest vascular plants was last revised in 1981 by Bacone et al. (3). Since that publication much additional field work has been undertaken and accordingly, our knowledge of Indiana's rarest plant species has greatly increased. The results of some of this extensive field work have been discussed by Homoya (9) and Homoya and Abrell (10) for southern Indiana and by Aldrich et al. (1) for northern Indiana. Wilhelm's (17) discussion on the special vegetation of the Indiana Dunes Na- tional Lakeshore has also provided us with a much clearer understanding of the status of rare, threatened and endangered plants in northwest Indiana. Unfortunately, the number of species thought to be extinct in Indiana has more than tripled. Previous reports (2, 3) indicated that 26 species were extirpated in In- diana. The work that has been conducted to date leads us to believe that as many as 90 species may be extirpated. Without a doubt, the single factor most responsible for this extirpation has been and continues to be, the destruction of natural habitat. Compilation and Selection Criteria Lists of Bacone and Hedge (2), Bacone et al. (3), Barnes (4), Crankshaw (5) and Crovello (6) were consulted and provide the foundation for this report. New additions to this list include state records discovered since Deam (7) and a number of recommen- dations from field botanists. -

Task Force on Landscape Heritage and Plant Diversity Has Determined Initial Designations
THE UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL TASK FORCE ON LANDSCAPE HERITAGE & PLANT DIVERSITY nd 2 EDITION APPROVED BY THE CHANCELLORS BUILDINGS AND GROUNDS COMMITTEE February, 2005 This report is the product of a more than one-year-long effort from concerned members of the University of North Carolina community to ensure that the culturally, historically, and ecologically significant trees and landscaped spaces of the Chapel Hill campus are preserved and maintained in a manner befitting their beauty and grandeur. At the time of this writing, Carolina is in the middle of the most significant building and renovation period in its history. Such a program poses many significant challenges to the survival and well-being of our cherished trees and landscapes. This report attempts to identify, promote awareness, and provide guidelines for both the protection and enhancement of the grounds of the University of North Carolina at Chapel Hill. Furthermore, this report is intended to work within the framework of two earlier documents that help guide development of the campus: the 2002 UNC Master Plan and the 1997 Report of the Chancellor’s Task Force on Intellectual Climate at UNC. We hope that members of the university community as well as outside consultants and contractors will find this information both useful and pertinent. The Taskforce on Landscape Heritage and Plant Diversity 1 This report is the product of a more than one-year-long effort from concerned members of the University of North Carolina community to ensure that the culturally, historically, and ecologically significant trees and landscaped spaces of the Chapel Hill campus are preserved and maintained in a manner befitting their beauty and grandeur. -
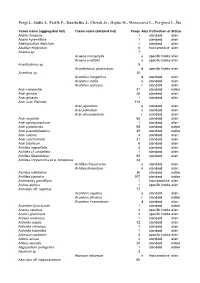
Pergl J., Sádlo J., Petřík P., Danihelka J., Chrtek Jr., Hejda M., Moravcová L., Perglová I., Štajerová K
Pergl J., Sádlo J., Petřík P., Danihelka J., Chrtek Jr., Hejda M., Moravcová L., Perglová I., Štajerová K. & Pyšek P. (2016): Dark side of the fence: ornamental plants as a source of wildgrowing flora in the Czech Republic. – Preslia 88: 163–184. Taxon name (aggregated list) Taxon name (detailed list) FrequencyAbundanceCultivation within demands aggregatedStatus taxa Abelia chinensis 1 standard alien Abelia ×grandiflora 1 standard alien Abeliophyllum distichum 1 standard alien Abutilon ×hybridum 6 frost sensitive alien Acaena sp. 7 Acaena microphylla e specific habitatalien Acaena ovalifolia e specific habitatalien Acantholimon sp. 9 Acantholimon glumaceum d specific habitatalien Acanthus sp. 10 Acanthus hungaricus d standard alien Acanthus mollis e standard alien Acanthus spinosus r standard alien Acer campestre 31 standard native Acer ginnala 28 standard alien Acer griseum 1 standard alien Acer sect. Palmata 113 Acer japonicum e standard alien Acer palmatum e standard alien Acer shirasawanum r standard alien Acer negundo 55 standard alien Acer pennsylvanicum 1 standard alien Acer platanoides 68 standard native Acer pseudoplatanus 39 standard native Acer rubrum 3 standard alien Acer saccharinum 21 standard alien Acer tataricum 6 standard alien Achillea ageratifolia 3 standard alien Achillea cf. umbellata 1 standard alien Achillea filipendulina 83 standard alien Achillea chrysocoma et A. tomentosa 23 Achillea chrysocoma e standard alien Achillea tomentosa e standard alien Achillea millefolium 36 standard native Achillea ptarmica 107 standard -

Bulletin of the Natural History Museum
Bulletin of _ The Natural History Bfit-RSH MU8&M PRIteifTBD QENERAl LIBRARY Botany Series VOLUME 23 NUMBER 2 25 NOVEMBER 1993 The Bulletin of The Natural History Museum (formerly: Bulletin of the British Museum (Natural History)), instituted in 1949, is issued in four scientific series, Botany, Entomology, Geology (incorporating Mineralogy) and Zoology. The Botany Series is edited in the Museum's Department of Botany Keeper of Botany: Dr S. Blackmore Editor of Bulletin: Dr R. Huxley Assistant Editor: Mrs M.J. West Papers in the Bulletin are primarily the results of research carried out on the unique and ever- growing collections of the Museum, both by the scientific staff and by specialists from elsewhere who make use of the Museum's resources. Many of the papers are works of reference that will remain indispensable for years to come. All papers submitted for publication are subjected to external peer review for acceptance. A volume contains about 160 pages, made up by two numbers, published in the Spring and Autumn. Subscriptions may be placed for one or more of the series on an annual basis. Individual numbers and back numbers can be purchased and a Bulletin catalogue, by series, is available. Orders and enquiries should be sent to: Intercept Ltd. P.O. Box 716 Andover Hampshire SPIO lYG Telephone: (0264) 334748 Fax: (0264) 334058 WorW Lwr abbreviation: Bull. nat. Hist. Mus. Lond. (Bot.) © The Natural History Museum, 1993 Botany Series ISSN 0968-0446 Vol. 23, No. 2, pp. 55-177 The Natural History Museum Cromwell Road London SW7 5BD Issued 25 November 1993 Typeset by Ann Buchan (Typesetters), Middlesex Printed in Great Britain at The Alden Press.