SEROLOGICAL TECHNIQUES – I (Secondary Binding Tests)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Syllabus for M
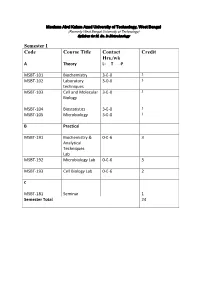
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -

Comparison of Immunohistochemistry with Immunoassay (ELISA
British Journal of Cancer (1999) 79(9/10), 1534–1541 © 1999 Cancer Research Campaign Article no. bjoc.1998.0245 Comparison of immunohistochemistry with immunoassay (ELISA) for the detection of components of the plasminogen activation system in human tumour tissue CM Ferrier1, HH de Witte2, H Straatman3, DH van Tienoven2, WL van Geloof1, FJR Rietveld1, CGJ Sweep2, DJ Ruiter1 and GNP van Muijen1 Departments of 1Pathology, 2Chemical Endocrinology and 3Epidemiology, University Hospital Nijmegen, PO Box 9101, 6500 HB Nijmegen, The Netherlands Summary Enzyme-linked immunosorbent assay (ELISA) methods and immunohistochemistry (IHC) are techniques that provide information on protein expression in tissue samples. Both methods have been used to investigate the impact of the plasminogen activation (PA) system in cancer. In the present paper we first compared the expression levels of uPA, tPA, PAI-1 and uPAR in a compound group consisting of 33 cancer lesions of various origin (breast, lung, colon, cervix and melanoma) as quantitated by ELISA and semi-quantitated by IHC. Secondly, the same kind of comparison was performed on a group of 23 melanoma lesions and a group of 28 breast carcinoma lesions. The two techniques were applied to adjacent parts of the same frozen tissue sample, enabling the comparison of results obtained on material of almost identical composition. Spearman correlation coefficients between IHC results and ELISA results for uPA, tPA, PAI-1 and uPAR varied between 0.41 and 0.78, and were higher for the compound group and the breast cancer group than for the melanoma group. Although a higher IHC score category was always associated with an increased median ELISA value, there was an overlap of ELISA values from different scoring classes. -

Immunoassay - Elisa
IMMUNOASSAY - ELISA PHUBETH YA-UMPHAN National Institute of Health, Department of Medical Sciences 0bjective After this presentation, participants will be able to Explain how an ELISA test determines if a person has certain antigens or antibodies . Explain the process of conducting an ELISA test. Explain interactions that take place at the molecular level (inside the microtiter well) during an ELISA test. Outline - Principal of immunoassay - Classification of immunoassay Type of ELISA - ELISA ELISA Reagents General - Applications Principal of ELISA ELISA workflow What is immunoassay? Immunoassays are bioanalytical methods that use the specificity of an antigen-antibody reaction to detect and quantify target molecules in biological samples. Specific antigen-antibody recognition Principal of immunoassay • Immunoassays rely on the inherent ability of an antibody to bind to the specific structure of a molecule. • In addition to the binding of an antibody to its antigen, the other key feature of all immunoassays is a means to produce a measurable signal in response to the binding. Classification of Immunoassays Immunoassays can be classified in various ways. Unlabeled Labeled Competitive Homogeneous Noncompetitive Competitive Heterogeneous Noncompetitive https://www.sciencedirect.com/science/article/pii/S0075753508705618 Classification of Immunoassays • Unlabeled - Immunoprecipitation • Labeled Precipitation of large cross-linked Ag-Ab complexes can be visible to the naked eyes. - Fluoroimmnoassay (FIA) - Radioimmunoassay (RIA) - Enzyme Immunoassays (EIA) - Chemiluminescenceimmunoassay(CLIA) - Colloidal Gold Immunochromatographic Assay (ICA) https://www.creative-diagnostics.com/Immunoassay.htm Classification of Immunoassays • Homogeneous immunoassays Immunoassays that do not require separation of the bound Ag-Ab complex. (Does not require wash steps to separate reactants.) Example: Home pregnancy test. • Heterogeneous immunoassays Immunoassays that require separation of the bound Ag-Ab complex. -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

Laboratory Techniques Used for Immunological Laboratory Methods
Laboratory techniques used for Immunological laboratory methods Dr. Tatiana Jones, MD, PhD NCC How to Make Serial Dilutions? Interpretation can be made differently depending on the nature of test. For example, if we need to figure out in what sample the concentration of the antibody or antigen is higher, we will go by TITER, which is the lowest serial dilution (let’s say that it is 1:32 in the picture on the left) that gives us positive result. This mean that even diluted 32 times sample is still capable of reacting. The other scenario when we are interpreting quantitative assays, such as ELISA. In this case we need to match results of our samples to known concentrations of STANDARD and MULTIPLY be our dilution factor. What is Antibody Titer? An antibody titer is a measurement of how much antibody an organism has produced that recognizes a particular antigen. Titer is expressed as the inverse of the greatest dilution that still gives a positive result. ELISA is a common means of determining antibody titers. How to Determine Antibody Titer? Where we can use Indirect Coombs test detects the presence of anti-Rh antibodies in blood serum. A patient might be reported to have an "indirect Antibody Titer? Coombs titer" of 16. This means that the patient's serum gives a positive indirect Coombs test at any dilution down to 1/16 (1 part serum to 15 parts diluent). At greater dilutions the indirect Coombs test is negative. If a few weeks later the same patient had an indirect Coombs titer of 32 (1/32 dilution which is 1 part serum to 31 parts diluent), this would mean that more anti-Rh antibody was made, since it took a greater dilution to eradicate the positive test. -

Radial Immunodiffusion Assay Protocol
Radial Immunodiffusion Aim: To study the immunodiffusion technique by Single Radial Immunodiffusion. Introduction: Single Radial Immunodiffusion, also known as Mancini technique, is a quantitative immunodiffusion technique used to detect the concentration of antigen by measuring the diameter of the precipitin ring formed by the interaction of the antigen and the antibody at optimal concentration. In this method the antibody is incorporated into the agarose gel whereas the antigen diffuses into it in a radial pattern. Thus, the antibody is uniformly distributed throughout the gel. Principle: Single Radial Immunodiffusion is used extensively for the quantitative estimation of antigen. Here the antigen-antibody reaction is made more sensitive by the addition of antiserum into the agarose gel and loading the antigen sample in the well. As the antigen diffuses into the agarose radially in all directions, it’s concentration continuously falls until the equivalence point is reached at which the antigen concentration is in equal proportion to that of the antibody present in the agarose gel. At this point ring of precipitation (‘precipitin ring’) is formed around the well. The diameter of the precipitin ring is proportional to the concentration of antigen. With increasing concentration of antigen, precipitin rings with larger diameter are formed. The size of the precipitin rings depends on: Antigen concentration in the sample well Antibody concentration in the agarose gel Size of the sample well Volume of the sample Thus, by having various concentrations of a standard antigen, standard curve can be obtained from which one can determine the amount of an antigen in an unknown sample. Thus, this is a quantitative test. -

An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1981 An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate Allen H. Smith Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Biochemistry Commons Recommended Citation Smith, Allen H., "An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate" (1981). All Graduate Theses and Dissertations. 5295. https://digitalcommons.usu.edu/etd/5295 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. AN ENZYME- LINKED ThltviUNOSORBE!'IT ASSAY (ELISA) FOR PANTOTHENATE by Allen H. Smith A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biochemistry UTAH STATE UNIVERSITY Logan, Utah 1981 ii ACKNOWLEDGEMENTS To Dr. R. G. Hansen, to Dr. B. W. Wyse, to Carl Wittwer, to Jack Brown, to Jan Pearson, to Nedra Christensen, to all those who have made this experience one of tremendous growth, I express my thanks. I express appreciation to the United States Department of Agriculture, under Grant #5901-0410-9-0288-0 with Utah State University, for financial support. Finally, I express thanks to my parents, who have come to realize that graduate school is also a part of life. Allen H. Smith "iii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ii LIST OF TABLES . vi LIST OF FIGURES. vii · ABSTRACT .. ix INTRODUCTION 1 REVIEW OF LITERATURE 4 Pantothenate Assays . -

Serotyping of Bdellovibrios by Agglutination and Indirect Immunofluorescence
INTERNATIONAL JOURNAL OF SYSTEMATICBACT~RIOLOGY. Oct. 1983, p. 816-821 Vol. 33, No. 4 0020-7713/83/040816-06$02.00/0 Copyright 0 1983, Internationnl Union of Microbiologicdl Societie\ Serotyping of Bdellovibrios by Agglutination and Indirect Immunofluorescence M. E. SCHELLING’* AND S. F. CONTI’ Department Biology, Texus A &M Unitwsit?, College Station, Texus 77843‘ und University of Mussa ch us et t s , A rn ii erst, Muss N c h iiset t J 0 1 002’ A comparative serological study was undertaken to clarify the uncertain interrelationships among various bdellovibrios. A total of 17 predacious (host- dependent) strains and 12 nonpredacious strains were serotyped. Strains were grouped into serotypes on the basis of positive agglutination and strongly positive fluorescence cross-reactions. The results of division of the strains into serogroups by these two methods were identical. Bdellovihrio starrii strain A3.12 and Bdellovibrio stolpii strain UKi2 were found to be antigenically distinct from each other and from the group of strains comprising Bdellovihrio hacteriovorus; the latter was found to be an antigenically heterogeneous group consisting of at least nine serogroups. All nonpredacious strains were found to be related antigenically to their obligately predacious counterparts. An antigen(s) common to all of the Bdellovibrio strains examined was exhibited as weakly positive fluorescence. Selected strains of Bdellovihrio were studied by Ouchterlony immunodiffusion, which confirmed the serogrouping results and provided a technically simple method of serotyping. Further immunochemical characterization of the strains of Bdellovihrio in conjunction with increased knowledge of other differences among strains will likely result in the formation of additional species of Bdellovihrio. -

Red Blood Cell Preparation and Hemagglutination Assay
Red Blood Cell Preparation and Hemagglutination Assay Purpose: This protocol describes the preparation of RBCs for storage and use in Hemagluttination assays, which are used to determine the La Sota B1 lentogenic Newcastle Disease virion hemaglutinin-neuraminidase titer relative to virion stock positive controls. The protocol was adapted from McGinnes et al. (2006) and “Detection of Hemagglutinating Viruses” (n.d.). Materials: ● Whole Blood Cells ● 50mL conical tube ● Multichannel micropipette, micropipette, and tips ● PBS with 2mM Penicillin/Streptomycin ● SV3 ● Alsever’s Solution ● 96 round bottom well plate ● Microscope slide ● Virion stock ● Centrifuge Procedure: 1. RBC preparation a. Obtain 25mL whole blood and add 25mL cold Alsever’s Solution for anti-coagulation in a 50mL conical tube and keep on ice b. Centrifuge whole blood at 500 RCF for 10min c. Aspirate blood plasma, buffy layer, and top erythrocytes d. Wash 3 times by resuspending in PBS with 2mM Penicillin/Streptomycin to double the pellet volume, centrifuging at 500 RCF, and aspirating the supernatant e. Resuspend the pellet in PBS with 2mM Penicillin/Streptomycin or SV3 for a final concentration of 10% pellet volume per total volume, and store at 2-7˚C for 1 week or 42 days 2. HA Titer a. Thaw your virion stock and samples on ice b. Use a multichannel micropipette to add 50µL of cold PBS to each row on a 96 round bottom well plate for each sample in duplicates c. Add 50µL of each sample to column 1 of their respective duplicate rows i. Don’t add anything to the negative control rows and add virion stocks to the positive control rows d. -

Detection of Virus-Specific Immunoglobulins Using a Doubly Labeled Fluorescein- 125I Antibody A
JOURNAL OF CLINICAL MICROBIOLOGY, June 1976, p. 637-639 Vol. 3, No. 6 Copyright © 1976 American Society for Microbiology Printed in U.S.A. Detection of Virus-Specific Immunoglobulins Using a Doubly Labeled Fluorescein- 125I Antibody A. J. PARKINSON* AND J. KALMAKOFF Department ofMicrobiology, University of Otago, Dunedin, New Zealand Received for publication 17 February 1976 Commercially prepared fluorescein-labeled antihuman antibodies were la- beled with 125I and used to compare specific herpes simplex virus antibody titers as determined by indirect fluorescent antibody and radioimmunoassay tech- niques. Total virus-specific immunoglobulin and virus-specific immunoglobulin G titers did not vary by more than one twofold dilution when compared by the two methods. Efforts are being made to develop a reliable calf serum, penicillin (100 U/ml) streptomycin radioimmunoassay (RIA) for the detection of (100 ,ug/ml), and 0.1% bicarbonate, were in- virus-specific immunoglobulins, acceptable for fected with the isolated virus. Uninoculated use in diagnostic serology (1, 2, 5-8). The estab- monolayers were maintained as controls. When lishment of a satisfactory RIA depends on the infected monolayers showed 75% cytopathic use of antibody with both a high avidity and effect, both inoculated and uninoculated cells selectivity for the material to be assayed (4). were dispersed, using 0.015% ethylenediamine- Consequently, a major obstacle to using RIA tetraacetic acid. Both cellular suspensions were routinely is the necessity of preparing specific standardized to contain 2.5 x 105 cells/ml in high-titer antibody against human immuno- phosphate-buffered saline. Using 0.025-ml vol- globulins (IgG, IgM, and IgA). -

Immune Effector Mechanisms and Designer Vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA
EXPERT REVIEW OF VACCINES https://doi.org/10.1080/14760584.2019.1674144 REVIEW How vaccines work: immune effector mechanisms and designer vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Three major advances have led to increase in length and quality of human life: Received 6 June 2019 increased food production, improved sanitation and induction of specific adaptive immune Accepted 25 September 2019 responses to infectious agents (vaccination). Which has had the most impact is subject to debate. KEYWORDS The number and variety of infections agents and the mechanisms that they have evolved to allow Vaccines; immune effector them to colonize humans remained mysterious and confusing until the last 50 years. Since then mechanisms; toxin science has developed complex and largely successful ways to immunize against many of these neutralization; receptor infections. blockade; anaphylactic Areas covered: Six specific immune defense mechanisms have been identified. neutralization, cytolytic, reactions; antibody- immune complex, anaphylactic, T-cytotoxicity, and delayed hypersensitivity. The role of each of these mediated cytolysis; immune immune effector mechanisms in immune responses induced by vaccination against specific infectious complex reactions; T-cell- mediated cytotoxicity; agents is the subject of this review. delayed hypersensitivity Expertopinion: In the past development of specific vaccines for infections agents was largely by trial and error. With an understanding of the natural history of an infection and the effective immune response to it, one can select the method of vaccination that will elicit the appropriate immune effector mechanisms (designer vaccines). These may act to prevent infection (prevention) or eliminate an established on ongoing infection (therapeutic). -

Importance of Ag-Ab Reactions
Ag-Ab reactions Tests for Ag-Ab reactions EISA SALEHI PhD. Immunology Dept. TUMS Importance of Ag-Ab Reactions • Understand the mechanisms of defense • Abs as tools in: – Treatment – Diagnosis • As biomarkers • As tools to measure analytes Nature of Ag/Ab Reactions http://www.med.sc.edu:85/chime2/lyso-abfr.htm • Lock and Key Concept • Non-covalent Bonds – Hydrogen bonds – Electrostatic bonds – Van der Waal forces – Hydrophobic bonds • Multiple Bonds • Reversible Source: Li, Y., Li, H., Smith-Gill, S. J., Mariuzza, R. A., Biochemistry 39, 6296, 2000 Affinity • Strength of the reaction between a single antigenic determinant and a single Ab combining site High Affinity Low Affinity Ab Ab Ag Ag Affinity = ( attractive and repulsive forces Calculation of Affinity Ag + Ab ↔ Ag-Ab Applying the Law of Mass Action: [[gAg-Ab] Keq = [Ag] x [Ab] Avidity • The overall strength of binding between an Ag with many determinants and multivalent Abs 4 6 10 Keq = 10 10 10 Affinity Avidity Avidity SifiitSpecificity • The ability of an individual antibody combining site to react with only one antigenic determinant. • The ability of a population of antibody molecules to react with only one antigen. Cross Reactivity • The ability of an individual Ab combining site to react with more than one antigenic determinant. • The ability of a population of Ab molecules to react with more than one Ag Cross reactions Anti-A Anti-A Anti-A Ab Ab Ab Ag A Ag B Ag C Shared epitope Similar epitope Factors Affecting Measurement of A/AbRAg/Ab Reac tions • Affinity • Avidity Ab excess Ag excess • AAbiAg:Ab ratio •Phyygsical form of Ag Equivalence – Lattice formation Do you need to know what happens in Lab.