CALLISTOPHYTALEAN PTERIDOSPERMS from PERMIAN AGED FLORAS of CHINA by LEYLA J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fossils and Plant Phylogeny1
American Journal of Botany 91(10): 1683±1699. 2004. FOSSILS AND PLANT PHYLOGENY1 PETER R. CRANE,2,5 PATRICK HERENDEEN,3 AND ELSE MARIE FRIIS4 2Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK; 3Department of Biological Sciences, The George Washington University, Washington DC 20052 USA; 4Department of Palaeobotany, Swedish Museum of Natural History, Box 50007, S-104 05 Stockholm, Sweden Developing a detailed estimate of plant phylogeny is the key ®rst step toward a more sophisticated and particularized understanding of plant evolution. At many levels in the hierarchy of plant life, it will be impossible to develop an adequate understanding of plant phylogeny without taking into account the additional diversity provided by fossil plants. This is especially the case for relatively deep divergences among extant lineages that have a long evolutionary history and in which much of the relevant diversity has been lost by extinction. In such circumstances, attempts to integrate data and interpretations from extant and fossil plants stand the best chance of success. For this to be possible, what will be required is meticulous and thorough descriptions of fossil material, thoughtful and rigorous analysis of characters, and careful comparison of extant and fossil taxa, as a basis for determining their systematic relationships. Key words: angiosperms; fossils; paleobotany; phylogeny; spermatophytes; tracheophytes. Most biological processes, such as reproduction or growth distic context, neither fossils nor their stratigraphic position and development, can only be studied directly or manipulated have any special role in inferring phylogeny, and although experimentally using living organisms. Nevertheless, much of more complex models have been developed (see Fisher, 1994; what we have inferred about the large-scale processes of plant Huelsenbeck, 1994), these have not been widely adopted. -

System Klasyfikacji Organizmów
1 SYSTEM KLASYFIKACJI ORGANIZMÓW Nie ma dziś ogólnie przyjętego systemu klasyfikacji organizmów. Nie udaje się osiągnąć zgodności nie tylko w odniesieniu do zakresów i rang poszczególnych taksonów, ale nawet co do zasad metodologicznych, na których klasyfikacja powinna być oparta. Zespołowe dzieła przeglądowe z reguły prezentują odmienne i wzajemnie sprzeczne podejścia poszczególnych autorów, bez nadziei na consensus. Nie ma więc mowy o skompilowaniu jednolitego schematu klasyfikacji z literatury i system przedstawiony poniżej nie daje nadziei na zaakceptowanie przez kogokolwiek poza kompilatorem. Przygotowany został w oparciu o kilka zasad (skądinąd bardzo kontrowersyjnych): (1) Identyfikowanie ga- tunków i ich klasyfikowanie w jednostki rodzajowe, rodzinowe czy rzędy jest zadaniem specjalistów i bez szcze- gółowych samodzielnych studiów nie można kwestionować wyników takich badań. (2) Podział świata żywego na królestwa, typy, gromady i rzędy jest natomiast domeną ewolucjonistów i dydaktyków. Powody, które posłużyły do wydzielenia jednostek powinny być jasno przedstawialne i zrozumiałe również dla niespecjalistów, albowiem (3) podstawowym zadaniem systematyki jest ułatwianie laikom i początkującym badaczom poruszanie się w obezwładniającej złożoności świata żywego. Wątpliwe jednak, by wystarczyło to do stworzenia zadowalającej klasyfikacji. W takiej sytuacji można jedynie przypomnieć, że lepszy ułomny system, niż żaden. Królestwo PROKARYOTA Chatton, 1938 DNA wyłącznie w postaci kolistej (genoforów), transkrypcja nie rozdzielona przestrzennie od translacji – rybosomy w tym samym przedzia- le komórki, co DNA. Oddział CYANOPHYTA Smith, 1938 (Myxophyta Cohn, 1875, Cyanobacteria Stanier, 1973) Stosunkowo duże komórki, dwuwarstwowa błona (Gram-ujemne), wewnętrzna warstwa mureinowa. Klasa CYANOPHYCEAE Sachs, 1874 Rząd Stigonematales Geitler, 1925; zigen – dziś Chlorofil a na pojedynczych tylakoidach. Nitkowate, rozgałęziające się, cytoplazmatyczne połączenia mię- Rząd Chroococcales Wettstein, 1924; 2,1 Ga – dziś dzy komórkami, miewają heterocysty. -

Retallack 2021 Coal Balls
Palaeogeography, Palaeoclimatology, Palaeoecology 564 (2021) 110185 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Modern analogs reveal the origin of Carboniferous coal balls Gregory Retallack * Department of Earth Science, University of Oregon, Eugene, Oregon 97403-1272, USA ARTICLE INFO ABSTRACT Keywords: Coal balls are calcareous peats with cellular permineralization invaluable for understanding the anatomy of Coal ball Pennsylvanian and Permian fossil plants. Two distinct kinds of coal balls are here recognized in both Holocene Histosol and Pennsylvanian calcareous Histosols. Respirogenic calcite coal balls have arrays of calcite δ18O and δ13C like Carbon isotopes those of desert soil calcic horizons reflecting isotopic composition of CO2 gas from an aerobic microbiome. Permineralization Methanogenic calcite coal balls in contrast have invariant δ18O for a range of δ13C, and formed with anaerobic microbiomes in soil solutions with bicarbonate formed by methane oxidation and sugar fermentation. Respiro genic coal balls are described from Holocene peats in Eight Mile Creek South Australia, and noted from Carboniferous coals near Penistone, Yorkshire. Methanogenic coal balls are described from Carboniferous coals at Berryville (Illinois) and Steubenville (Ohio), Paleocene lignites of Sutton (Alaska), Eocene lignites of Axel Heiberg Island (Nunavut), Pleistocene peats of Konya (Turkey), and Holocene peats of Gramigne di Bando (Italy). Soils and paleosols with coal balls are neither common nor extinct, but were formed by two distinct soil microbiomes. 1. Introduction and Royer, 2019). Although best known from Euramerican coal mea sures of Pennsylvanian age (Greb et al., 1999; Raymond et al., 2012, Coal balls were best defined by Seward (1895, p. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -

A Physiologically Explicit Morphospace for Tracheid-Based Water Transport in Modern and Extinct Seed Plants
A Physiologically Explicit Morphospace for Tracheid-based Water Transport in Modern and Extinct Seed Plants The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Wilson, Jonathan P., and Andrew H. Knoll. 2010. A physiologically explicit morphospace for tracheid-based water transport in modern and extinct seed plants. Paleobiology 36(2): 335-355. Published Version doi:10.1666/08071.1 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:4795216 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP Wilson - 1 A Physiologically Explicit Morphospace for Tracheid-Based Water Transport in Modern and Extinct Seed Plants Jonathan P. Wilson* Andrew H. Knoll September 7, 2009 RRH: PHYSIOLOGICALLY EXPLICIT MORPHOSPACE LRH: JONATHAN P. WILSON AND ANDREW H. KNOLL Wilson - 2 Abstract We present a morphometric analysis of water transport cells within a physiologically explicit three-dimensional space. Previous work has shown that cell length, diameter, and pit resistance govern the hydraulic resistance of individual conducting cells; thus, we use these three parameters as axes for our morphospace. We compare living and extinct plants within this space to investigate how patterns of plant conductivity have changed over evolutionary time. Extinct coniferophytes fall within the range of living conifers, despite differences in tracheid-level anatomy. Living cycads, Ginkgo biloba, the Miocene fossil Ginkgo beckii, and extinct cycadeoids overlap with both conifers and vesselless angiosperms. -

Syllabus M.Sc. Botany (Choice Based Credit System)
Syllabus M.Sc. Botany (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) DEPARTMENT OF BOTANY UNIVERSITY OF ALLAHABAD Page 1 of 21 DEPARTMENT OF BOTANY UNIVERSITY OF ALLAHABAD M.Sc. Syllabus (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) Semester – I Course Code Marks Course Title Credits BOT501 100 Phycology and Limnology 3 BOT502 100 Mycology and Plant Pathology 3 BOT503 100 Bryology and Pteridology 3 BOT504 100 Gymnosperm and Palaeobotany 3 BOT531 100 Lab Work I (based on Course BOT501 and BOT502) 4 (Excursion/field work/ Project) BOT532 100 Lab Work II (based on Course BOT503 and BOT504) 4 (Excursion/field work/Project) Total credits 20 Semester – II Course Code Marks Course Title Credits BOT505 100 Plant Morphology and Anatomy 3 BOT506 100 Reproductive Biology, Morphogenesis and Tissue culture 3 BOT507 100 Taxonomy of Angiosperm and Economic Botany 3 BOT508 100 Ecology and Phytogeography 3 BOT533 100 Lab Work III (based on Course BOT505 and BOT506) 4 (Field work/ Project) BOT534 100 Lab Work IV (based on Course BOT507 and BOT508) 4 (Field work/ Project) Total credits 20 Semester – III Course Code Marks Course Title Credits BOT601 100 Plant Physiology 3 BOT602 100 Plant Biochemistry and Biochemical Techniques 3 BOT603 100 Cytogenetics, Plant Breeding and Biostatistics 3 BOT604 100 Microbiology 3 BOT631 100 Lab Work V (based on Course BOT601 and BOT602) 4 BOT632 100 Lab Work VI (based on Course BOT603 and BOT604) 4 Total credits 20 Semester – IV Course Code Marks Course -

CPY Document
CHAPTER 1 Plant diversity has evolved and is evolving on the earth today according to the processes of natural selection. All basic "body plans" of vascular plants were in existence by the Early Carboniferous, approximately 340 million years ago, and each major radiation occupied a particular habitat type, for example, wetland versus terra firma, pristine versus disturbed environments. Today the great spe- cies diversity we see on theplanetis dominated by just a few of these hasicplant types. This chapter sets the stage to understanding the origin and radiation of plant diversity on the earth before the significant influence of humans. The im- plication is that plants may become more diverse in the number of species level over time, but less diverse in overall form. The history of life tells us that plants that are dominant and diverse today will certainly be inconspicuous or even ex--' tinct in the distant future. EVOLUTION OF LAND PLANT DIVERSITY MAJOR INNOVATIONS AND LINEAGES THROUGH TIME William A. DiMichele and Richard M. Bateman PLANT DIVERSITY VIEWED through the lens of deep time takes on a con- siderably different aspect than when examined in the present. Although the fossil record captures only fragments of the terrestrial world of the past 450 million years, it does indicate clearly that the world of today is simply a passing phase, the latest permutation in a string of spasmodic changes in the ecological organization of the terrestrial biosphere. The pace of change in species diversity and that of ecosystem structure and composition have followed broadly parallel paths, unquestionably related but not always changing in unison. -

Curriculum Vitae
CURRICULUM VITAE ORCID ID: 0000-0003-0186-6546 Gar W. Rothwell Edwin and Ruth Kennedy Distinguished Professor Emeritus Department of Environmental and Plant Biology Porter Hall 401E T: 740 593 1129 Ohio University F: 740 593 1130 Athens, OH 45701 E: [email protected] also Courtesy Professor Department of Botany and PlantPathology Oregon State University T: 541 737- 5252 Corvallis, OR 97331 E: [email protected] Education Ph.D.,1973 University of Alberta (Botany) M.S., 1969 University of Illinois, Chicago (Biology) B.A., 1966 Central Washington University (Biology) Academic Awards and Honors 2018 International Organisation of Palaeobotany lifetime Honorary Membership 2014 Fellow of the Paleontological Society 2009 Distinguished Fellow of the Botanical Society of America 2004 Ohio University Distinguished Professor 2002 Michael A. Cichan Award, Botanical Society of America 1999-2004 Ohio University Presidential Research Scholar in Biomedical and Life Sciences 1993 Edgar T. Wherry Award, Botanical Society of America 1991-1992 Outstanding Graduate Faculty Award, Ohio University 1982-1983 Chairman, Paleobotanical Section, Botanical Society of America 1972-1973 University of Alberta Dissertation Fellow 1971 Paleobotanical (Isabel Cookson) Award, Botanical Society of America Positions Held 2011-present Courtesy Professor of Botany and Plant Pathology, Oregon State University 2008-2009 Visiting Senior Researcher, University of Alberta 2004-present Edwin and Ruth Kennedy Distinguished Professor of Environmental and Plant Biology, Ohio -

Transformative Paleobotany
Chapter 6 Lower Permian Flora of the Sanzenbacher Ranch, Clay County, Texas William A. DiMichele1, Robert W. Hook2, Hans Kerp3, Carol L. Hotton1,4, Cindy V. Looy5 and Dan S. Chaney1 1NMNH Smithsonian Institution, Washington, DC, United States; 2The University of Texas at Austin, Austin, TX, United States; 3Westfälische Wilhelms-Universität Münster, Münster, Germany; 4National Institutes of Health, Bethesda, MD, United States; 5University of California Berkeley, Berkeley, CA, United States 1. INTRODUCTION 1985; Broutin, 1986; Popa, 1999; Steyer et al., 2000; Wagner and Mayoral, 2007; Bercovici and Broutin, 2008; Since 1989, field parties supported by the U.S. National Barthel, 2009; Wagner and Álvarez-Vázquez, 2010; Museum of Natural History have obtained large collections Barthel and Brauner, 2015). Furthermore, because this of mainly Permian plant fossils from north central Texas. locality was collected on three occasions over a time period This work was undertaken to study known localities and to of 50 years and by different parties, comparative analysis of find new fossiliferous deposits that would contribute to a the Sanzenbacher collections provides a basis for assessing better understanding of floral and paleoenvironmental sites that have comparable histories. changes within the region during the early Permian. From the outset, the effort was interdisciplinary and grew, through the contributions of nearly 20 paleobotanists, 2. GEOLOGY palynologists, invertebrate and vertebrate paleontologists, Clay County is the only county in the Permo-Carboniferous and sedimentary geologists of several subdisciplines, to be outcrop belt of north central Texas that lacks marine rocks. quite comprehensive. Our reporting of results, however, has These alluvial sediments accumulated east of a broad been influenced by unexpected developments, including the coastal plain that bordered the Eastern Shelf of the Midland discovery of new plant-fossil assemblages in areas once Basin. -

Petrified Pennsylvanian Age Plants of Eastern Ohio1
PETRIFIED PENNSYLVANIAN AGE PLANTS OF EASTERN OHIO1 GAR W. ROTHWELL, Department of Botany, Ohio University, Athens, Ohio 45701 Abstract. The recent (1975) discovery of coal-ball petrifactions in the Duquesne and Ames coals of the Conemaugh Group provides an opportunity to make detailed studies of abundant and well preserved Pennsylvanian age fossil plants. Material from these beds was collected at a single location west of Steubenville, Ohio. Remains assignable to all the major groups of coal swamp plants were present, with the psaroniaceous tree ferns and medullosan seed ferns most abundantly represented. To date, 50 distinct taxa of plant remains have been discovered. OHIO J. SCI. 76(3): 128, 1976 Pennsylvanian age plants, preserved by calcareous cellular permineralization (coal balls), are among the most valuable of Paleozoic fossils. At localities where Duquesne large quantities of material are available for study, features such as anatomical structure and plant habit can often be thoroughly examined (e.g. Dennis, 1974). In instances where preservation is espe- cially good, developmental sequences and even reproductive mechanisms sometimes can be interpreted (e.g. Millay and Eg- gert, 1974). Unfortunately, known col- lecting localities of coal balls are few in number. In the Appalachian Basin only six discoveries have thus far been re- ported (Cross, 1967; Schopf, 1961); in- cluding two in Ohio (Good, personal communication; Good and Taylor, 1974). It is therefore of considerable importance that two new Ohio coal-ball localities are described. LOCALITIES AND STRATIGRAPHY Two coal seams were exposed in a road cut on the south side of Ohio Route 22 (NE M SE K SE 14, Sec. -
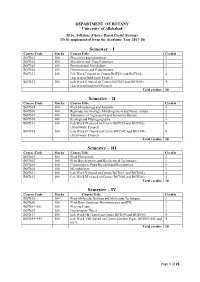
Semester – I Semester – II Semester – III Semester – IV
DEPARTMENT OF BOTANY University of Allahabad M.Sc. Syllabus (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) Semester – I Course Code Marks Course Title Credits BOT501 100 Phycology and Limnology 3 BOT502 100 Mycology and Plant Pathology 3 BOT503 100 Bryology and Pteridology 3 BOT504 100 Gymnosperm and Palaeobotany 3 BOT531 100 Lab Work I (based on Course BOT501 and BOT502) 4 (Excursion/field work/ Project) BOT532 100 Lab Work II (based on Course BOT503 and BOT504) 4 (Excursion/field work/Project) Total credits 20 Semester – II Course Code Marks Course Title Credits BOT505 100 Plant Morphology and Anatomy 3 BOT506 100 Reproductive Biology, Morphogenesis and Tissue culture 3 BOT507 100 Taxonomy of Angiosperm and Economic Botany 3 BOT508 100 Ecology and Phytogeography 3 BOT533 100 Lab Work III (based on Course BOT505 and BOT506) 4 (Field work/ Project) BOT534 100 Lab Work IV (based on Course BOT507 and BOT508) 4 (Field work/ Project) Total credits 20 Semester – III Course Code Marks Course Title Credits BOT601 100 Plant Physiology 3 BOT602 100 Plant Biochemistry and Biochemical Techniques 3 BOT603 100 Cytogenetics, Plant Breeding and Biostatistics 3 BOT604 100 Microbiology 3 BOT631 100 Lab Work V (based on Course BOT601 and BOT602) 4 BOT632 100 Lab Work VI (based on Course BOT603 and BOT604) 4 Total credits 20 Semester – IV Course Code Marks Course Title Credits BOT605 100 Plant Molecular Biology and Molecular Techniques 3 BOT606 100 Plant Biotechnology, Bioinformatics and IPR 3 BOT651-662 100 Elective Paper -

Coal Author(S): William A
Stem and Leaf Cuticle of Karinopteris: Source of Cuticles from the Indiana "Paper" Coal Author(s): William A. DiMichele, Michael O. Rischbieter, Donald L. Eggert and Robert A. Gastaldo Reviewed work(s): Source: American Journal of Botany, Vol. 71, No. 5 (May - Jun., 1984), pp. 626-637 Published by: Botanical Society of America Stable URL: http://www.jstor.org/stable/2443359 . Accessed: 31/10/2012 10:50 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Botanical Society of America is collaborating with JSTOR to digitize, preserve and extend access to American Journal of Botany. http://www.jstor.org Amer. J. Bot. 71(5): 626-637. 1984. STEM AND LEAF CUTICLE OF KARINOPTERIS: SOURCE OF CUTICLES FROM THE INDIANA "PAPER" COAL1 WILLIAMA. DIMICHELESMICHAEL O. RISCHBIETER, DONALD L. EGGERT,AND ROBERTA. GASTALDO Departmentof Botany, University of Washington,Seattle, Washington; Department of Biology, WesternIllinois University, Macomb, Illinois; IndianaGeological Survey, Bloomington, Indiana,and Departmentof Geology, AuburnUniversity, Auburn,Alabama ABSTRACT Cuticularor "paper"coal-shale is a local deposit of an organic-rich,highly clastic rock, with abundantleafand stem cuticles,associated with the UpperBlock Coal Member in ParkeCounty, Indiana.Fresh blocks of cuticularcoal can be split alongbedding surfaces to reveala fossil flora of low diversity, dominatedby pteridospermsand lycopods, with minor amounts of ferns and sphenopsids.Karinopteris is a subdominantcomponent of this flora and the great abundance of well-preservedcuticles of this plant allows for a reconstructionof its frondand growthhabit.