Lawrence Berkeley National Laboratory Recent Work
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

United States Patent 0 " Ice Patented May 28, 1968
3,385,666 United States Patent 0 " ice Patented May 28, 1968 1 2 of novel compositions that can be used as a source of 3,385,666 both ozone and oxygen. DIOXYGENYL FLUORIDES OF GROUP V An additional object of this invention is the preparation ELEMENTS of the nitronium ion and intermediates for preparing Archie R. Young II, Montclair, Tetsuyuki Hirata, Whar novel nitronium salts. ton, and Scott I. Morrow, Morris Plains, N.J., assign Further objects of this invention will become apparent ors to Thiokol Chemical Corporation, Bristol, Pa., a corporation of Delaware to the reader upon a further reading of this patent appli No Drawing. Filed Jan. 6, 1964, Ser. No. 336,061 cation. 11 Claims. (Cl. 23-203) The above objects among many others are achieved by 10 the direct interaction of certain ?uoride reactants with This invention relates to a novel class of ?uorinated, dioxygen di?uoride at reaction temperatures well below oxygen containing, oxidizing agents and to a process for 0° C. As indicated earlier the ?uoride reactant is selected their preparation. from the group consisting of Group V ?uorides. More particularly, this invention concerns the prepara The preferred practice is to contact the ?uoride reactant tion of certain stable ?uorides of the cationic dioxygenyl 15 with an excess of 02112 at low temperatures ranging from radical. These novel-oxidizing agents have the formula: —l60 to \—78° C. until a substantial amount of prod uct is formed. The product is isolated after evacuating off the excess 021:2, ?uorine and gaseous by-products. wherein (O2) is the dioxygenyl radical having a charge In one ‘favored process embodiment of this invention of +1, M is an element selected from the group consist 20 a ?uoride reactant chosen ‘from the preferred phosphorus, ing of phosphorus, arsenic antimony and bismuth. -

Proceedings of the Nuclear Energy Agency International Workshop on Chemical Hazards in Fuel Cycle Facilities Nuclear Processing
Nuclear Safety NEA/ CSNI/R(2019)9/ADD1 May 2019 www.oecd-nea.org Proceedings of the Nuclear Energy Agency International Workshop on Chemical Hazards in Fuel Cycle Facilities Nuclear Processing Appendix C NEA/CSNI/R(2019)9/ADD1 The non-radiological risks involving dangerous chemicals in nuclear fuel cycle facilities - French framework regulation - Youcef HEMIMOU, Brice DELIME, Raffaello AMOROSI, Josquin VERNON French Nuclear Safety Authority 15, rue Louis Lejeune CS70013 – 92541 Montrouge Cedex [email protected] Accidents involving hazardous chemicals pose a significant threat to the population and the environment. Among nuclear facilities, this threat applies in particular to the fuel cycle facilities. As a consequence, the activities related to hazardous chemicals are covered by a legal framework that, depending on the nature of the activity and the associated risks, aims to guarantee that, they will not be likely to be detrimental to safety, public health and environment [1]. The aim of this article is to present a summary of the main regulations involving dangerous chemicals in nuclear fuel cycle facilities that have to be taken into account in the safety demonstration. The latter must prove that the risks of an accident - radiological or not - and the scale of its consequences, given the current state of knowledge, practices and the vulnerability of the installation environment, are as low as possible under acceptable economic conditions. Key words : nuclear fuel cycle facilities, safety demonstration, principle of defence in depth, deterministic approach, probabilistic approach, dangerous chemicals, non-radiological risks, Seveso Directive, domino effects. 1. THE DANGEROUS SUBSTANCES IN NUCLEAR FUEL CYCLE FACILITIES 1.1. -

Chemical Redox Agents for Organometallic Chemistry
Chem. Rev. 1996, 96, 877−910 877 Chemical Redox Agents for Organometallic Chemistry Neil G. Connelly*,† and William E. Geiger*,‡ School of Chemistry, University of Bristol, U.K., and Department of Chemistry, University of Vermont, Burlington, Vermont 05405-0125 Received October 3, 1995 (Revised Manuscript Received January 9, 1996) Contents I. Introduction 877 A. Scope of the Review 877 B. Benefits of Redox Agents: Comparison with 878 Electrochemical Methods 1. Advantages of Chemical Redox Agents 878 2. Disadvantages of Chemical Redox Agents 879 C. Potentials in Nonaqueous Solvents 879 D. Reversible vs Irreversible ET Reagents 879 E. Categorization of Reagent Strength 881 II. Oxidants 881 A. Inorganic 881 1. Metal and Metal Complex Oxidants 881 2. Main Group Oxidants 887 B. Organic 891 The authors (Bill Geiger, left; Neil Connelly, right) have been at the forefront of organometallic electrochemistry for more than 20 years and have had 1. Radical Cations 891 a long-standing and fruitful collaboration. 2. Carbocations 893 3. Cyanocarbons and Related Electron-Rich 894 Neil Connelly took his B.Sc. (1966) and Ph.D. (1969, under the direction Compounds of Jon McCleverty) degrees at the University of Sheffield, U.K. Post- 4. Quinones 895 doctoral work at the Universities of Wisconsin (with Lawrence F. Dahl) 5. Other Organic Oxidants 896 and Cambridge (with Brian Johnson and Jack Lewis) was followed by an appointment at the University of Bristol (Lectureship, 1971; D.Sc. degree, III. Reductants 896 1973; Readership 1975). His research interests are centered on synthetic A. Inorganic 896 and structural studies of redox-active organometallic and coordination 1. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Coordination Chemistry of Xenon
Coordination Chemistry of Xenon Paul Griffin Literature Seminar 11/14/19 Situated on the far side of the periodic table, the chemical inertness of the noble gases has led to their widespread applications ranging from gas-discharge lamps and ion engines for space travel to medical applications including as MRI contrast agents and radiotherapy.1 Though all practical applications of the noble gases center around Xe (0), understanding the reactivity and bonding of xenon in higher oxidation states is fundamental to investigating chemistry at the far end of the periodic table. The reactivity of the noble gases, in particular xenon, was first uncovered when Bartlett, who had previously prepared dioxygenyl hexafluoroplatinate (V) (O2PtF6), noted similar ionization energies for molecular oxygen and atomic xenon (a difference of 0.1 eV).2 Mixing of xenon with PtF6 vapor led to the formation of an orange-yellow solid led to the synthesis of the first noble gas compound, xenon hexafluoroplatinate (V) (XePtF6). The chemistry of xenon has evolved significantly since its’ discovery to include an assortment of xenon fluorides, oxides, oxylfluorides, organoxenon3, and metalloxenon compounds such as the tetraxenoaurate (II) cation (Figure 1).4 These species have weak covalent bonding between Xe and the oxygen/fluorine moieties.5 The chemistry of xenon is dominated by the 2+, 4+, and 6+ oxidation states, though Xe (VIII) compounds are known in the form of perxenates and xenon tetroxide. These primarily ionic species, particularly the oxides, oxylfluorides, and fluorides, serve as precursors to noncovalent adducts of xenon. Figure 1. Organoxenon and metalloxenon species Recent work has led to the preparation of xenon adducts with noncovalently interacting ligands such as acetonitrile6a, 6b, ketones6c, sulfoxides6c, and crown ethers6d (Figure 2) capable of weakly coordinating to xenon salts to afford stable species at room temperature. -

Novel Contributions to the Solid-State Chemistry of Diazenides” 05/2009–11/2009 Master Thesis (Inorganic Chemistry) Ludwig-Maximilian University Munich (Prof
Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Novel Contributions to the Solid-State Chemistry of Diazenides Sebastian Bernhard Schneider aus München, Deutschland 2013 Erklärung Diese Dissertation wurde im Sinne von § 7 der Promotionsordnung vom 28. November 2011 von Herrn Prof. Dr. Wolfgang Schnick betreut. Eidesstattliche Versicherung Diese Dissertation wurde eigenständig und ohne unerlaubte Hilfe erarbeitet. München, 11.11.2013 …………………………………… (Sebastian Bernhard Schneider) Dissertation eingereicht am 11.11.2013 1. Gutachter: Prof. Dr. Wolfgang Schnick 2. Gutachter: Prof. Dr. Dirk Johrendt Mündliche Prüfung am 16.12.2013 To my family ACKNOWLEDGEMENTS First, I would like to express my gratitude to my supervisor Prof. Dr. Wolfgang Schnick for the opportunity to do my PhD under his guidance. Throughout the entire work, I appreciated his extraordinary expertise, the given freedom in research and writing and the support at any time. For being the co-referee of my thesis, I thank Prof. Dr. Dirk Johrendt. I am thankful to Prof. Dr. Konstantin Karaghiosoff, PD Dr. Markus Lackinger, Prof. Dr. Hans-Christian Böttcher, and Prof. Dr. Jörn Schmedt a. d. Günne for being available as examiners in my viva-voce. In addition, I have to thank various co-workers spread all over Germany and Europe. Hereby, special thanks go to • Volker L. Deringer, Dr. Ralf P. Stoffel as well as Prof. Dr. Richard Dronskowski (RWTH Aachen, Germany) for their long-lasting support with numerous theoretical calculations concerning novel diazenide compounds and for their ongoing enthusiasm until we found the final ground state. • Dr. Lkhamsuren Bayarjargal, Dr. -

Reducing Surface Recombination Velocity in Thin Perovskite Films
Thermochemistry of Ruthenium in Molten Fluoride Salts Metal or Melt? by Laurens G. Braskamp in partial fulfillment of the requirements for the degree of Bachelor of Science in Molecular Science and Technology at the Delft University of Technology to be defended publicly on Monday, August 20, 2018 at 14:30 Student numbers: 4411064 (TU Delft) 1517988 (Universiteit Leiden) Thesis Committee: dr. Elisa Capelli (supervisor) TU Delft dr. Anna L. Smith prof. dr. Rudy J.M. Konings Abstract The Molten Salt Reactor (MSR) is a member of safer and more sustainable fourth generation of nuclear reactors, the development of the might be crucial to continually secure supply of electrical energy from the near future onwards. Because the fluorides of ruthenium (44Ru) are a poorly investigated group of fission products that might be born during MSR operation, some computational research was done on their thermochemistry. Because little experimental data on these species has been obtained, the gas-phase molecular geometries and formation enthalpies of all known Ru-F binary compounds, RuF, RuF2, RuF3, RuF4, RuF5, RuF6, (RuF5)2 and (RuF5)3 are calculated by Density Functional Theory. The DFT/B3LYP method was employed with quasi-relativistic ECP28MWB pseudopotentials and basis set on ruthenium and cc-pVTZ basis set on fluorine. Standard molar entropies and heat capacities were then also calculated by statistical mechanics computations. From the obtained thermochemical data, part of which had to be optimized, and additional data on the solid and liquid phases, the Ru-F subsystem was assessed using the CALPHAD methodology. Based on the developed model, a phase diagram, Ellingham diagram and gas-phase equilibrium diagram were calculated. -

Lawrence Berkeley Laboratory UNIVERSITY of CALIFORNIA Materials & Molecular Research Division
u.c- '+ LBL-20810 Rev. c.\ Preprint Lawrence Berkeley Laboratory UNIVERSITY OF CALIFORNIA Materials & Molecular Research Division . '_ ') 1986 :sRARy AND ~·-- ........ _ Submitted to the Journal of the American Chemical Society THE PREPARATION AND CHARACTERIZATION OF RADICAL CATION SALTS DERIVED FROM PERFLUOROBENZENE, PERFLUOROTOLUENE AND PERFLUORONAPHTHALENE T.J. Richardson, F.L. Tanzella and N. Bartlett April 1986 For Reference Not to be taken from this room Prepared for the U.S. Department of Energy under Contract DE-AC03-76SF00098 " ~ .<. DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain conect information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any wananty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. LBL-20810 The Preparation and Characterization of Radical-Cation Salts Derived from Perfluorobenzene, Perfluorotoluene and Perfluoronaphthalene T. J. Richardson, F. L. -
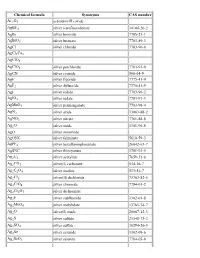
List of Chemical Formulas
Chemical formula Synonyms CAS number Ac2O3 actinium(III) oxide AgBF4 silver tetrafluoroborate 14104-20-2 AgBr silver bromide 7785-23-1 AgBrO3 silver bromate 7783-89-3 AgCl silver chloride 7783-90-6 AgCl3Cu2 AgClO3 AgClO4 silver perchlorate 7783-93-9 AgCN silver cyanide 506-64-9 AgF silver fluoride 7775-41-9 AgF2 silver difluoride 7775-41-9 AgI silver iodide 7783-96-2 AgIO3 silver iodate 7783-97-3 AgMnO4 silver permanganate 7783-98-4 AgN3 silver azide 13863-88-2 AgNO3 silver nitrate 7761-88-8 Ag2O silver oxide 1301-96-8 AgO silver monoxide AgONC silver fulminate 5610-59-3 AgPF6 silver hexafluorophosphate 26042-63-7 AgSNC silver thiocyanate 1701-93-5 Ag2C2 silver acetylide 7659-31-6 Ag2CO3 silver(I) carbonate 534-16-7 Ag2C2O4 silver oxalate 533-51-7 Ag2Cl2 silver(II) dichloride 75763-82-5 Ag2CrO4 silver chromate 7784-01-2 Ag2Cr2O7 silver dichromate Ag2F silver subfluoride 1302-01-8 Ag2MoO4 silver molybdate 13765-74-7 Ag2O silver(I) oxide 20667-12-3 Ag2S silver sulfide 21548-73-2 Ag2SO4 silver sulfate 10294-26-5 Ag2Se silver selenide 1302-09-6 Ag2SeO3 silver selenite 7784-05-6 Ag2SeO4 silver selenate 7784-07-8 Ag2Te silver(I) telluride 12002-99-2 Ag3Br2 silver dibromide 11078-32-3 Ag3Br3 silver tribromide 11078-33-4 Ag3Cl3 silver(III) trichloride 12444-96-1 Ag3I3 silver(III) triiodide 37375-12-5 Ag3PO4 silver phosphate 7784-09-0 AlBO aluminium boron oxide 12041-48-4 AlBO2 aluminium borate 61279-70-7 AlBr aluminium monobromide 22359-97-3 AlBr3 aluminium tribromide 7727-15-3 AlCl aluminium monochloride 13595-81-8 AlClF aluminium chloride fluoride -

Chapter 5 Molecular Orbitals
Chapter 5 Molecular Orbitals Molecular orbital theory uses group theory to describe the bonding in molecules ; it comple- ments and extends the introductory bonding models in Chapter 3 . In molecular orbital theory the symmetry properties and relative energies of atomic orbitals determine how these orbitals interact to form molecular orbitals. The molecular orbitals are then occupied by the available electrons according to the same rules used for atomic orbitals as described in Sections 2.2.3 and 2.2.4 . The total energy of the electrons in the molecular orbitals is compared with the initial total energy of electrons in the atomic orbitals. If the total energy of the electrons in the molecular orbitals is less than in the atomic orbitals, the molecule is stable relative to the separate atoms; if not, the molecule is unstable and predicted not to form. We will first describe the bonding, or lack of it, in the first 10 homonuclear diatomic molecules ( H2 through Ne2 ) and then expand the discussion to heteronuclear diatomic molecules and molecules having more than two atoms. A less rigorous pictorial approach is adequate to describe bonding in many small mole- cules and can provide clues to more complete descriptions of bonding in larger ones. A more elaborate approach, based on symmetry and employing group theory, is essential to under- stand orbital interactions in more complex molecular structures. In this chapter, we describe the pictorial approach and develop the symmetry methodology required for complex cases. 5.1 Formation of Molecular Orbitals from Atomic Orbitals As with atomic orbitals, Schrödinger equations can be written for electrons in molecules. -

"Noble-Gas Chemistry and the Periodic System of Mendeleev" By
"Noble-Gas Chemistry and The Periodic System of Mendeleev" By Neil Bartlett Materials and Molecular Research Division, Lawrence Berkeley Laboratory and Department of Chemistry, University of California Berkeley, California U.S.A. The Stable Noble-Gas Electron Configuration When, in 1962, chemists were informed that a compound of a noble- gas had been prepared', there was much expression of surprise and ini- tially even disbelief. Faith in the chemical inertness of the noble gases had been fostered in part by previous failures to prepare compounds. The greatest prejudice, however, derived from the electronic theories of the chemical bond, which stressed the noble-gas electron arrangement as the ideal to which all other atoms tended. When the noble gases were discovered2, in the last years of the 19th Century, they were quickly recognized as a new Group of elements of Mende- * leev's Table of The Elements. This new Group of elements fitted naturally into the "Table", each noble-gas being located .between a halogen and an alkali metal. Since the Halogens included the most strongly oxidizing elements, whereas the Alkali Metals were the most strongly reducing ele- ments of the Periodic Table, it was appropriate, for the intervening group of elements, to exhibit neither oxidizing nor reducing properties, i.e. to be chemically unreactive. All efforts to oxidize or reduce helium and argon' (i.e. to bring them into chemical combination with other elements) failed!, perhaps the most significant failure being Moissan's attempt in 1895 to prepare an argon fluoride3. The rarer noble gases were not sub- jected to the same intensive chemical investigation, and no claim for chemical activity of the gases was sustained prior to 1962.