Identification of Biomarkers in Macrophages of Atherosclerosis By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PARSANA-DISSERTATION-2020.Pdf
DECIPHERING TRANSCRIPTIONAL PATTERNS OF GENE REGULATION: A COMPUTATIONAL APPROACH by Princy Parsana A dissertation submitted to The Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland July, 2020 © 2020 Princy Parsana All rights reserved Abstract With rapid advancements in sequencing technology, we now have the ability to sequence the entire human genome, and to quantify expression of tens of thousands of genes from hundreds of individuals. This provides an extraordinary opportunity to learn phenotype relevant genomic patterns that can improve our understanding of molecular and cellular processes underlying a trait. The high dimensional nature of genomic data presents a range of computational and statistical challenges. This dissertation presents a compilation of projects that were driven by the motivation to efficiently capture gene regulatory patterns in the human transcriptome, while addressing statistical and computational challenges that accompany this data. We attempt to address two major difficulties in this domain: a) artifacts and noise in transcriptomic data, andb) limited statistical power. First, we present our work on investigating the effect of artifactual variation in gene expression data and its impact on trans-eQTL discovery. Here we performed an in-depth analysis of diverse pre-recorded covariates and latent confounders to understand their contribution to heterogeneity in gene expression measurements. Next, we discovered 673 trans-eQTLs across 16 human tissues using v6 data from the Genotype Tissue Expression (GTEx) project. Finally, we characterized two trait-associated trans-eQTLs; one in Skeletal Muscle and another in Thyroid. Second, we present a principal component based residualization method to correct gene expression measurements prior to reconstruction of co-expression networks. -

CDH12 Cadherin 12, Type 2 N-Cadherin 2 RPL5 Ribosomal
5 6 6 5 . 4 2 1 1 1 2 4 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 2 2 A A A A A A A A A A A A A A A A A A A A C C C C C C C C C C C C C C C C C C C C R R R R R R R R R R R R R R R R R R R R B , B B B B B B B B B B B B B B B B B B B , 9 , , , , 4 , , 3 0 , , , , , , , , 6 2 , , 5 , 0 8 6 4 , 7 5 7 0 2 8 9 1 3 3 3 1 1 7 5 0 4 1 4 0 7 1 0 2 0 6 7 8 0 2 5 7 8 0 3 8 5 4 9 0 1 0 8 8 3 5 6 7 4 7 9 5 2 1 1 8 2 2 1 7 9 6 2 1 7 1 1 0 4 5 3 5 8 9 1 0 0 4 2 5 0 8 1 4 1 6 9 0 0 6 3 6 9 1 0 9 0 3 8 1 3 5 6 3 6 0 4 2 6 1 0 1 2 1 9 9 7 9 5 7 1 5 8 9 8 8 2 1 9 9 1 1 1 9 6 9 8 9 7 8 4 5 8 8 6 4 8 1 1 2 8 6 2 7 9 8 3 5 4 3 2 1 7 9 5 3 1 3 2 1 2 9 5 1 1 1 1 1 1 5 9 5 3 2 6 3 4 1 3 1 1 4 1 4 1 7 1 3 4 3 2 7 6 4 2 7 2 1 2 1 5 1 6 3 5 6 1 3 6 4 7 1 6 5 1 1 4 1 6 1 7 6 4 7 e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m -

KDELR3 Dnaxpab a C-Terminal Tetrapeptide Signal, Usually Lys-Asp-Glu-Leu (KDEL) in Animal Cells, and His-Asp-Glu-Leu (HDEL) in S
KDELR3 DNAxPab a C-terminal tetrapeptide signal, usually lys-asp-glu-leu (KDEL) in animal cells, and his-asp-glu-leu (HDEL) in S. Catalog Number: H00011015-W01P cerevisiae. This process is mediated by a receptor that recognizes, and binds the tetrapeptide-containing Regulation Status: For research use only (RUO) protein, and returns it to the ER. In yeast, the sorting receptor encoded by a single gene, ERD2, is a Product Description: Rabbit polyclonal antibody raised seven-transmembrane protein. Unlike yeast, several against a full-length human KDELR3 DNA using DNAx™ human homologs of the ERD2 gene, constituting the Immune technology. KDEL receptor gene family, have been described. KDELR3 was the third member of the family to be Immunogen: Full-length human DNA identified, and it encodes a protein highly homologous to KDELR1 and KDELR2 proteins. Two transcript variants Sequence: of KDELR3 that arise by alternative splicing, and encode MNVFRILGDLSHLLAMILLLGKIWRSKCCKGISGKSQIL different isoforms of KDELR3 receptor, have been FALVFTTRYLDLFTNFISIYNTVMKVVFLLCAYVTVYMIY described. [provided by RefSeq] GKFRKTFDSENDTFRLEFLLVPVIGLSFLENYSFTLLEIL WTFSIYLESVAILPQLFMISKTGEAETITTHYLFFLGLYR ALYLANWIRRYQTENFYDQIAVVSGVVQTIFYCDFFYL YVTKVLKGKKLSLPMPI Host: Rabbit Reactivity: Human Applications: Flow Cyt-Tr, IF-Ex, IF-Tr, WB-Tr (See our web site product page for detailed applications information) Protocols: See our web site at http://www.abnova.com/support/protocols.asp or product page for detailed protocols Purification: Protein A Storage Buffer: In 1x PBS, pH 7.4 Storage Instruction: Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Entrez GeneID: 11015 Gene Symbol: KDELR3 Gene Alias: ERD2L3 Gene Summary: Retention of resident soluble proteins in the lumen of the endoplasmic reticulum (ER) is achieved in both yeast and animal cells by their continual retrieval from the cis-Golgi, or a pre-Golgi compartment. -

Examining Differences of Ubiquitination on KDEL Receptor Isoform Expression Zachary Dixon Mentored by Dr
Examining differences of ubiquitination on KDEL receptor isoform expression Zachary Dixon Mentored by Dr. Emily Wires and Dr. Brandon Harvey Introduction Materials and Methods (cont.) Results (cont.) The disruption of vital cellular processes, commonly supported by Following the identification of putative PTMs (Figure 2), KDELrs were probed Flag Pull-Down Flow-Through the transport of proteins emanant from the endoplasmic reticulum for ubiquitination. SY5Y cells were plated in a 24–well plate, and KDELr plasmids (ER), is a primary factor in disease pathogenesis. Key proteins in this were transfected into cells with no additional treatment, vehicle, or a proteasomal R1 R2 R1 R2 R1 R2 R1 R1 R1 R1 R2 R2 R2 R2 R1 transport are KDEL receptors (KDELrs). Post-translation, nascent inhibitor (MG132). Cells were then lysed and collected for immunoprecipitation R2 MANF proteins are folded by ER chaperones, a subgroup of ER-resident (IP). IP experiments were performed with magnetic beads incubated with FLAG- MANF proteins (ERPs), and moved them to the ER membrane (Trychta et antibodies, followed by PBS-T washes. Samples were centrifuged, incubated with al., 2018). They may also undergo post-translational modifications cell culture media, and eluted. Samples were then run on a 4–12% Bis-Tris gel and 51 kDa (PTMs). Post-secretion from the ER, they are trafficked through the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. For IgG Golgi, and to their final destination. However, ERPs are unique in this analysis of KDELr isoform relative abundance, PVDF membranes were probed 39 kDa regard; ERPs return from the Golgi to the ER upon interaction with a with IR secondary antibodies and scanned with an infrared scanner. -

Co-Evolution of B7H6 and Nkp30, Identification of a New B7 Family Member, B7H7, and of B7's Historical Relationship with the MHC
Immunogenetics (2012) 64:571–590 DOI 10.1007/s00251-012-0616-2 ORIGINAL PAPER Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7's historical relationship with the MHC Martin F. Flajnik & Tereza Tlapakova & Michael F. Criscitiello & Vladimir Krylov & Yuko Ohta Received: 19 January 2012 /Accepted: 20 March 2012 /Published online: 11 April 2012 # Springer-Verlag 2012 Abstract The B7 family of genes is essential in the regula- Furthermore, we identified a Xenopus-specific B7 homolog tion of the adaptive immune system. Most B7 family mem- (B7HXen) and revealed its close linkage to B2M, which we bers contain both variable (V)- and constant (C)-type have demonstrated previously to have been originally domains of the immunoglobulin superfamily (IgSF). encoded in the MHC. Thus, our study provides further proof Through in silico screening of the Xenopus genome and that the B7 precursor was included in the proto MHC. subsequent phylogenetic analysis, we found novel genes Additionally, the comparative analysis revealed a new B7 belonging to the B7 family, one of which is the recently family member, B7H7, which was previously designated in discovered B7H6. Humans and rats have a single B7H6 the literature as an unknown gene, HHLA2. gene; however, many B7H6 genes were detected in a single large cluster in the Xenopus genome. The B7H6 expression Keywords B7 family. MHC . Evolution . Natural killer patterns also varied in a species-specific manner. Human receptors . Genetic linkage . Xenopus B7H6 binds to the activating natural killer receptor, NKp30. While the NKp30 gene is single-copy and maps to the MHC in most vertebrates, many Xenopus NKp30 genes were Introduction found in a cluster on a separate chromosome that does not harbor the MHC. -

Prenatal Detection of TAR Syndrome in a Fetus with Compound Inheritance of an RBM8A SNP and a 334‑Kb Deletion: a Case Report
MOLECULAR MEDICINE REPORTS 9: 163-165, 2014 Prenatal detection of TAR syndrome in a fetus with compound inheritance of an RBM8A SNP and a 334‑kb deletion: A case report IOANNIS PAPOULIDIS1, EIRINI OIKONOMIDOU1, SANDRO ORRU2, ELISAVET SIOMOU1, MARIA KONTODIOU1, MAKARIOS ELEFTHERIADES3, VASILIOS BACOULAS4, JUAN C. CIGUDOSA5, JAVIER SUELA5, LORETTA THOMAIDIS6 and EMMANOUIL MANOLAKOS1,2 1Laboratory of Genetics, Eurogenetica S.A., Thessaloniki 55133, Greece; 2Department of Medical Genetics, University of Cagliari, Binaghi Hospital, Cagliari I‑09126, Italy; 3Embryocare, Fetal Medicine Unit, Athens 11522; 4Fetal Medicine Centre, Athens 10674, Greece; 5NIMGenetics, Madrid 28049, Spain; 6Department of Pediatrics, Aglaia Kyriakou Children's Hospital, University of Athens, Athens 11527, Greece Received May 30, 2013; Accepted October 16, 2013 DOI: 10.3892/mmr.2013.1788 Abstract. Thrombocytopenia-absent radius syndrome identified the presence of a minimally deleted 200‑kb region (TAR) is a rare genetic disorder that is characterized by the at chromosome band 1q21.1 in patients with TAR, but it is not absence of the radius bone in each forearm and a markedly sufficient to cause the phenotype (3,4). A study identified two reduced platelet count that results in life-threatening bleeding rare single nucleotide polymorphisms (SNPs) in the regulatory episodes (thrombocytopenia). Tar syndrome has been associ- region of the RBM8A gene that are involved in TAR syndrome ated with a deletion of a segment of 1q21.1 cytoband. The through the reduction of the expression of the RBM8A-encoded 1q21.1 deletion syndrome phenotype includes Tar and other Y14 protein (4). The first allele (rs139428292 G>A), which is features such as mental retardation, autism and microcephaly. -

Genome-Wide Transcriptional Analysis of Carboplatin Response in Chemosensitive and Chemoresistant Ovarian Cancer Cells
1605 Genome-wide transcriptional analysis of carboplatin response in chemosensitive and chemoresistant ovarian cancer cells David Peters,1,2 John Freund,2 and Robert L. Ochs2 variety of disease processes, including cancer. Patterns of global gene expression can reveal the molecular pathways 1 Department of Pharmacology and Therapeutics, University of relevant to the disease process and identify potential new Liverpool, United Kingdom and 2Precision Therapeutics, Inc., Pittsburgh, Pennsylvania therapeutic targets. The use of this technology for the molecular classification of cancer was recently shown with the identification of an expression profile that was Abstract predictive of patient outcome for B-cell lymphoma (1). We have recently described an ex vivo chemoresponse In addition, this study showed that histologically similar assay for determining chemosensitivity in primary cultures tumors can be differentiated based on their gene expression of human tumors. In this study, we have extended these profiles. Ultimately, these unique patterns of gene expres- experiments in an effort to correlate chemoresponse data sion may be used as guidelines to direct different modes of with gene expression patterns at the level of transcription. therapy. Primary cultures of cells derived from ovarian carcinomas Although it is widely recognized that patients with the of individual patients (n = 6) were characterized using same histologic stage and grade of cancer respond to the ChemoFx assay and classified as either carboplatin therapies differently, few clinical tests can predict indi- sensitive (n = 3) or resistant (n = 3). Three representa- vidual patient responses. The next great challenge will be tive cultures of cells from each individual tumor were then to use the power of post-genomic technology, including subjected to Affymetrix gene chip analysis (n = 18) using microarray analyses, to correlate gene expression patterns U95A human gene chip arrays. -

The Function of KDEL Receptors As UPR Genes in Disease
International Journal of Molecular Sciences Review The Function of KDEL Receptors as UPR Genes in Disease Emily S. Wires *,†, Kathleen A. Trychta †, Lacey M. Kennedy † and Brandon K. Harvey *,† Molecular Mechanisms of Cellular Stress and Inflammation, Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD 21224, USA; [email protected] (K.A.T.); [email protected] (L.M.K.) * Correspondence: [email protected] (E.S.W.); [email protected] (B.K.H.) † These authors contributed equally. Abstract: The KDEL receptor retrieval pathway is essential for maintaining resident proteins in the endoplasmic reticulum (ER) lumen. ER resident proteins serve a variety of functions, including protein folding and maturation. Perturbations to the lumenal ER microenvironment, such as calcium depletion, can cause protein misfolding and activation of the unfolded protein response (UPR). Additionally, ER resident proteins are secreted from the cell by overwhelming the KDEL receptor retrieval pathway. Recent data show that KDEL receptors are also activated during the UPR through the IRE1/XBP1 signaling pathway as an adaptive response to cellular stress set forth to reduce the loss of ER resident proteins. This review will discuss the emerging connection between UPR activation and KDEL receptors as it pertains to ER proteostasis and disease states. Keywords: KDEL receptor; endoplasmic reticulum; unfolded protein response; ER resident proteins; disease; exodosis 1. Introduction Citation: Wires, E.S.; Trychta, K.A.; Kennedy, L.M.; Harvey, B.K. The The endoplasmic reticulum (ER) is a dynamic intracellular organelle integral to cel- Function of KDEL Receptors as UPR lular homeostasis. Although the ER plays critical roles in lipid synthesis [1], calcium Genes in Disease. -
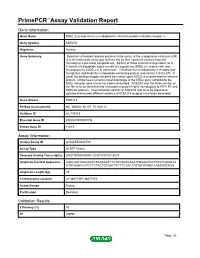
Download Validation Data
PrimePCR™Assay Validation Report Gene Information Gene Name KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 3 Gene Symbol KDELR3 Organism Human Gene Summary Retention of resident soluble proteins in the lumen of the endoplasmic reticulum (ER) is achieved in both yeast and animal cells by their continual retrieval from the cis-Golgi or a pre-Golgi compartment. Sorting of these proteins is dependent on a C-terminal tetrapeptide signal usually lys-asp-glu-leu (KDEL) in animal cells and his-asp-glu-leu (HDEL) in S. cerevisiae. This process is mediated by a receptor that recognizes and binds the tetrapeptide-containing protein and returns it to the ER. In yeast the sorting receptor encoded by a single gene ERD2 is a seven-transmembrane protein. Unlike yeast several human homologs of the ERD2 gene constituting the KDEL receptor gene family have been described. KDELR3 was the third member of the family to be identified and it encodes a protein highly homologous to KDELR1 and KDELR2 proteins. Two transcript variants of KDELR3 that arise by alternative splicing and encode different isoforms of KDELR3 receptor have been described. Gene Aliases ERD2L3 RefSeq Accession No. NC_000022.10, NT_011520.12 UniGene ID Hs.730819 Ensembl Gene ID ENSG00000100196 Entrez Gene ID 11015 Assay Information Unique Assay ID qHsaCED0042749 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000409006, ENST00000216014 Amplicon Context Sequence AGGCGGTACCAGACTGAGAATTTCTATGACCAAATTGCAGTCGTGTCTGGAGTA GTACAAACCATCTTCTACTGTGACTTCTTCTACTTGTATGTGACCAAAGGTAGG Amplicon Length (bp) 78 Chromosome Location 22:38877367-38877474 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 95 R2 0.9992 Page 1/5 PrimePCR™Assay Validation Report cDNA Cq 21.26 cDNA Tm (Celsius) 78.5 gDNA Cq 24.37 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. -

Table S1. 103 Ferroptosis-Related Genes Retrieved from the Genecards
Table S1. 103 ferroptosis-related genes retrieved from the GeneCards. Gene Symbol Description Category GPX4 Glutathione Peroxidase 4 Protein Coding AIFM2 Apoptosis Inducing Factor Mitochondria Associated 2 Protein Coding TP53 Tumor Protein P53 Protein Coding ACSL4 Acyl-CoA Synthetase Long Chain Family Member 4 Protein Coding SLC7A11 Solute Carrier Family 7 Member 11 Protein Coding VDAC2 Voltage Dependent Anion Channel 2 Protein Coding VDAC3 Voltage Dependent Anion Channel 3 Protein Coding ATG5 Autophagy Related 5 Protein Coding ATG7 Autophagy Related 7 Protein Coding NCOA4 Nuclear Receptor Coactivator 4 Protein Coding HMOX1 Heme Oxygenase 1 Protein Coding SLC3A2 Solute Carrier Family 3 Member 2 Protein Coding ALOX15 Arachidonate 15-Lipoxygenase Protein Coding BECN1 Beclin 1 Protein Coding PRKAA1 Protein Kinase AMP-Activated Catalytic Subunit Alpha 1 Protein Coding SAT1 Spermidine/Spermine N1-Acetyltransferase 1 Protein Coding NF2 Neurofibromin 2 Protein Coding YAP1 Yes1 Associated Transcriptional Regulator Protein Coding FTH1 Ferritin Heavy Chain 1 Protein Coding TF Transferrin Protein Coding TFRC Transferrin Receptor Protein Coding FTL Ferritin Light Chain Protein Coding CYBB Cytochrome B-245 Beta Chain Protein Coding GSS Glutathione Synthetase Protein Coding CP Ceruloplasmin Protein Coding PRNP Prion Protein Protein Coding SLC11A2 Solute Carrier Family 11 Member 2 Protein Coding SLC40A1 Solute Carrier Family 40 Member 1 Protein Coding STEAP3 STEAP3 Metalloreductase Protein Coding ACSL1 Acyl-CoA Synthetase Long Chain Family Member 1 Protein -

A Dissertation Entitled Role of Androgen Receptor in Folate
A Dissertation Entitled Role of Androgen Receptor in Folate Receptor α Regulation and in Prostate Cancer by Suneethi Sivakumaran Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biomedical Sciences ___________________________________________________________ Dr. Manohar Ratnam, Committee Chair ____________________________________________________________ Dr. Beata Lecka-Czernik, Committee Member ____________________________________________________________ Dr. Cynthia M. Smas, Committee Member ____________________________________________________________ Dr. Ivana De La Serna, Committee Member ____________________________________________________________ Dr. Lirim Shemshedini, Committee Member ____________________________________________________________ Dr. Robert J. Trumbly, Committee Member ____________________________________________________________ Dr. Patricia R. Komuniecki, Dean College of Graduate Studies The University of Toledo December, 2012 Copyright 2012, Suneethi Sivakumaran This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author An Abstract of Role of Androgen Receptor in Folate Receptor α Regulation and in Prostate Cancer by Suneethi Sivakumaran Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biomedical Sciences The University of Toledo June 2012 Folic acid is an essential water soluble vitamin required -

Melanoblast Transcriptome Analysis Reveals Novel Pathways Promoting Melanoma Metastasis
bioRxiv preprint doi: https://doi.org/10.1101/721712; this version posted August 6, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Melanoblast transcriptome analysis reveals novel pathways promoting melanoma metastasis Kerrie L. Marie1, Antonella Sassano1*, Howard H. Yang1*, Aleksandra M. Michalowski1, Helen T. Michael1, Theresa Guo1,2, Yien Che Tsai3, Allan M. Weissman3, Maxwell P. Lee1, Lisa M. Jenkins4, M. Raza Zaidi5, Eva Pérez-Guijarro1, Chi-Ping Day1, Heinz Arnheiter6, Sean Davis7, Paul S. Meltzer7, Glenn Merlino1** and Pravin J. Mishra1 1. Laboratory of Cancer Biology and Genetics, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. 2. Department of Otolaryngology - Head and Neck Surgery, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD 21287, USA. 3. Laboratory of Protein Dynamics and Signaling, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702, USA. 4. Laboratory of Cell Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. 5. Fels Institute for Cancer Research and Molecular Biology, Lewis Katz School of Medicine at Temple University, Philadelphia, PA 19140, USA. 6. Mammalian Development Section, National Institute of Neurological Disorders and Stroke, National Institute of Health, Bethesda, MD 20892, USA. 7. Genetics Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. * These authors contributed equally ** Corresponding author GM: Email – [email protected]; Phone – 240-760-6801 bioRxiv preprint doi: https://doi.org/10.1101/721712; this version posted August 6, 2019.