Transferability and Use of Microsatellite Markers for the Genetic Analysis of the Germplasm of Some Arachis Section Species of the Genus Arachis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AH16 Aboriginal and Torres Strait Islander Health
AH16 Aboriginal and Torres Strait Islander health www.racgp.org.au Healthy Profession. Healthy Australia. AH16 Aboriginal and Torres Strait Islander health Disclaimer The information set out in this publication is current at the date of first publication and is intended for use as a guide of a general nature only and may or may not be relevant to particular patients or circumstances. Nor is this publication exhaustive of the subject matter. Persons implementing any recommendations contained in this publication must exercise their own independent skill or judgement or seek appropriate professional advice relevant to their own particular circumstances when so doing. Compliance with any recommendations cannot of itself guarantee discharge of the duty of care owed to patients and others coming into contact with the health professional and the premises from which the health professional operates. Accordingly, The Royal Australian College of General Practitioners (RACGP) and its employees and agents shall have no liability (including without limitation liability by reason of negligence) to any users of the information contained in this publication for any loss or damage (consequential or otherwise), cost or expense incurred or arising by reason of any person using or relying on the information contained in this publication and whether caused by reason of any error, negligent act, omission or misrepresentation in the information. Recommended citation The Royal Australian College of General Practitioners. Curriculum for Australian General Practice 2016 – AH16 Aboriginal and Torres Strait Islander health. East Melbourne, Vic: RACGP, 2016. The Royal Australian College of General Practitioners 100 Wellington Parade East Melbourne, Victoria 3002 Australia Tel 03 8699 0510 Fax 03 9696 7511 www.racgp.org.au Published May 2016 © The Royal Australian College of General Practitioners We recognise the traditional custodians of the land and sea on which we work and live. -
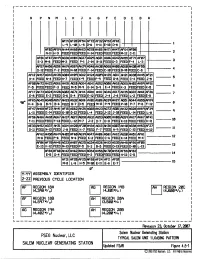
Salem Generating Station, Units 1 & 2, Revision 29 to Updated Final Safety Analysis Report, Chapter 4, Figures 4.5-1 to 4.5
r------------------------------------------- 1 I p M J B I R N L K H G F E D c A I I I I I Af'Jq AF20 AF54 AF72 32 AF52 AF18 I L-q L-10 L-15 D-6 -11 E-10 D-8 l I AF03 Af't;qAH44 AH60 AH63 AG70 AH65 AH7l AH47 AFS4 AF08 I N-ll H-3 FEED FEED FEED H-14 FEED FEED FEED M-12 C-11 2 I AF67 AH4q AH04 AG27 AG2<i' AG21 AG16 AG42 AF71 AF07 AF01 AG36 AH!5!5 3 I E-3 M-6 FEED M-3 FEED P-1 J-14 B-11 FEED D-3 FEED F-4 L-3 I AF67 AH5S AG56 Atflq AGsq AH2<1' AG48 AH30 AG68 AH08 AG60 AH30 AF55 I D-12 FEED F-2 FEED N-11 FEED F-14 FEED C-11 FEED B-11 FEED C-8 4 I AF12 AH57 AG43 AH38 AHtiJq AG12 AH24 AGfR AH25 AGil AG31 AH45 AF21 AGlM AH21 5 I H~4 FEED N-4 FEED H-7 FEED K~q FEED F-q FEED G-8 FEED C-4 FEED J-15 I AF50 AH72 AH22 AGS6 AH15 AGll.lAG64 AG41 AG52 AG88 AH18 AG65 AHIJ2 AH5q AF51 I F-5 FEED FEED F-3 FEED M-5 r+q G-14 o-q E-4 FEED K-3 FEED FEED K-5 6 I f:Fl7 AH73 AG24 AH28 AG82 AG71 AH14 AG18 AHil AG46 AG17 AH35 AG22 AH61 AF26 7 I E-8 FEED E-2 FEED G-6 G-4 FEED E-12 FEED J-4 J-6 FEED L-2 FEED E-5 I Af&q I qeo AF65 AG45 AtM0 AG57 AH33 AG32 AG16 AH01 AGI6 AG3<1' AH27 AG51 AG44 AG55 K-4 B-8 e-q B-6 FEED B-7 P-5 FEEC M-11 P-q FEED P-11 P-7 P-8 F-12 8 I AF47 AH68 AF23 AH41 AF1!5 AG62 AH26 AG03 AH23 AH32 AG28 AHsq AF3<1' q I L-U FEED E-14 FEED G-10 G-12 FEED L-4 FEED FEED L-14 FEED L-8 I ~~ AF66 AH66 AH10 AG67 AH37 AGJq AG68 AG3l AG63 AG05 AH08 AG5q AH17 AH67 AF41 I F-11 FEED FEED F-13 FEED L-12 M-7 J-2 D-7 D-11 FEED K-13 FEED FEED K-11 10 I AE33 AH!52 AG37 AH31 AG14 AH20 AF20 AH34 AG13 AH36 AG07 AH40 AG38 AH!53 AF27 I G-ll FEED N-12 FEED J-8 FEED K-7 FEED -

CADP 2.0) Infrastructure for Connectivity and Innovation
The Comprehensive Asia Development Plan 2.0 (CADP 2.0) Infrastructure for Connectivity and Innovation November 2015 Economic Research Institute for ASEAN and East Asia The findings, interpretations, and conclusions expressed herein do not necessarily reflect the views and policies of the Economic Research Institute for ASEAN and East Asia, its Governing Board, Academic Advisory Council, or the institutions and governments they represent. All rights reserved. Material in this publication may be freely quoted or reprinted with proper acknowledgement. Cover Art by Artmosphere ERIA Research Project Report 2014, No.4 National Library of Indonesia Cataloguing in Publication Data ISBN: 978-602-8660-88-4 Contents Acknowledgement iv List of Tables vi List of Figures and Graphics viii Executive Summary x Chapter 1 Development Strategies and CADP 2.0 1 Chapter 2 Infrastructure for Connectivity and Innovation: The 7 Conceptual Framework Chapter 3 The Quality of Infrastructure and Infrastructure 31 Projects Chapter 4 The Assessment of Industrialisation and Urbanisation 41 Chapter 5 Assessment of Soft and Hard Infrastructure 67 Development Chapter 6 Three Tiers of Soft and Hard Infrastructure 83 Development Chapter 7 Quantitative Assessment on Hard/Soft Infrastructure 117 Development: The Geographical Simulation Analysis for CADP 2.0 Appendix 1 List of Prospective Projects 151 Appendix 2 Non-Tariff Barriers in IDE/ERIA-GSM 183 References 185 iii Acknowledgements The original version of the Comprehensive Asia Development Plan (CADP) presents a grand spatial design of economic infrastructure and industrial placement in ASEAN and East Asia. Since the submission of such first version of the CADP to the East Asia Summit in 2010, ASEAN and East Asia have made significant achievements in developing hard infrastructure, enhancing connectivity, and participating in international production networks. -

Radiation, Protection of the Public and the Environment (Poster Session 1) Origin and Migration of Cs-137 in Jordanian Soils
Major scientific thematic areas: TA6 – Radiation, Protection of the Public and the Environment (Poster session 1) Origin and Migration of Cs-137 in Jordanian Soils Ahmed Qwasmeh, Helmut W. Fischer IUP- Institute for Environmental Physics, Bremen University, Germany Abstract Whilst some research and publication has been done and published about natural radioactivity in Jordan, only one paper has been published about artificial radioactivity in Jordanian soils (Al Hamarneh 2003). It reveals high concentrations of 137Cs and 90Sr in some regions in the northwest section of Jordan. The origin of this contamination was not determined. Two sets of soil samples were collected and brought from northwest section of Jordan for two reasons, namely; the comparable high concentration of 137Cs in this region according to the above-mentioned paper and because most of the population concentrates in this region. The first set of samples was collected in April 2004 from eleven different sites of this region of Jordan. The second set of samples has been brought in July 2005 from six of the previous sites where we had found higher 137Cs contamination. The second set was collected as thinner sliced soil samples for further studying and to apply a suitable model for 137Cs migration in soil. Activity of 137Cs was measured using a HpGe detector of 50% relative efficiency and having resolution of 2keV at 1.33MeV. Activity of 90Sr was measured for the samples of four sites of the first set of samples, using a gas-filled proportional detector with efficiency of 21.3% cps/Bq. The total inventory of 137Cs in Bq/m2 has been calculated and the correlation between 137Cs inventory and annual rainfall and site Altitude has been studied. -

1St IRF Asia Regional Congress & Exhibition
1st IRF Asia Regional Congress & Exhibition Bali, Indonesia November 17–19 , 2014 For Professionals. By Professionals. "Building the Trans-Asia Highway" Bali’s Mandara toll road Executive Summary International Road Federation Better Roads. Better World. 1 International Road Federation | Washington, D.C. ogether with the Ministry of Public Works Indonesia, we chose the theme “Building the Trans-Asia Highway” to bring new emphasis to a visionary project Tthat traces its roots back to 1959. This Congress brought the region’s stakeholders together to identify new and innovative resources to bridge the current financing gap, while also sharing case studies, best practices and new technologies that can all contribute to making the Trans-Asia Highway a reality. This Congress was a direct result of the IRF’s strategic vision to become the world’s leading industry knowledge platform to help countries everywhere progress towards safer, cleaner, more resilient and better connected transportation systems. The Congress was also a reflection of Indonesia’s rising global stature. Already the largest economy in Southeast Asia, Indonesia aims to be one of world’s leading economies, an achievement that will require the continued development of not just its own transportation network, but also that of its neighbors. Thank you for joining us in Bali for this landmark regional event. H.E. Eng. Abdullah A. Al-Mogbel IRF Chairman Minister of Transport, Kingdom of Saudi Arabia Indonesia Hosts the Region’s Premier Transportation Meeting Indonesia was the proud host to the 1st IRF Asia Regional Congress & Exhibition, a regional gathering of more than 700 transportation professionals from 52 countries — including Ministers, senior national and local government officials, academics, civil society organizations and industry leaders. -

Characterization and Transferability of Microsatellite Markers of the Cultivated Peanut (Arachis Hypogaea)
BMC Plant Biology BioMed Central Research article Open Access Characterization and transferability of microsatellite markers of the cultivated peanut (Arachis hypogaea) Marcos A Gimenes*1,2, Andrea A Hoshino1, Andrea VG Barbosa1, Dario A Palmieri1 and Catalina R Lopes1 Address: 1Laboratório de Biotecnologia e Genética Molecular (BIOGEM), Departamento de Genética, Instituto de Biociências, Universidade Estadual Paulista (UNESP), Botucatu, SP, Brazil and 2Instituto Agronômico de Campinas – RGV, Caixa Postal 28, Campinas, SP, Brazil Email: Marcos A Gimenes* - [email protected]; Andrea A Hoshino - [email protected]; Andrea VG Barbosa - [email protected]; Dario A Palmieri - [email protected]; Catalina R Lopes - [email protected] * Corresponding author Published: 27 February 2007 Received: 3 March 2006 Accepted: 27 February 2007 BMC Plant Biology 2007, 7:9 doi:10.1186/1471-2229-7-9 This article is available from: http://www.biomedcentral.com/1471-2229/7/9 © 2007 Gimenes et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: The genus Arachis includes Arachis hypogaea (cultivated peanut) and wild species that are used in peanut breeding or as forage. Molecular markers have been employed in several studies of this genus, but microsatellite markers have only been used in few investigations. Microsatellites are very informative and are useful to assess genetic variability, analyze mating systems and in genetic mapping. The objectives of this study were to develop A. -

BELLA COOLA to FOUR MILE TRAIL
BELLA COOLA to FOUR MILE TRAIL Trail Location & Engineering Design Project sponsored by Bella Coola General Hospital Central Coast Regional District & Union of BC Municipalities December 14, 2009 PO Box 216, Hagensborg, BC V0T 1H0 Tel:250-982-2515, [email protected] BC-4Mile Trail Layout Report -i- TABLE OF CONTENTS 1 INTRODUCTION 1 1.1 Layout & Survey Method 1 1.2 Trail Design Criteria 1 2 TRAIL LAYOUT & DESCRIPTION 1 2.1 Cut and Fill 2 2.2 Partial Fill 2 2.3 Overland Fill 3 2.4 Flush Surfacing 3 2.5 Detailed Description 4 2.6 Tatsquan Creek Crossing Options 5 2.6.1 Option A - Hwy 20 Sidewalk 5 2.6.2 Option A2 – Widened Sidewalk on Hwy Bridge 5 2.6.3 Option B – Parallel Footbridge 6 2.6.4 Option C – Downstream Footbridge 7 3 ENVIRONMENT 8 3.1 Fish 8 3.2 Wildlife 8 4 FIELD REVIEW 9 5 CONSTRUCTION 9 5.1 Trail Components 11 5.1.1 Asphalt 11 5.1.2 Crush Gravel 11 5.1.3 Sub-grade Ballast 11 5.1.4 Foot Bridges 11 5.1.5 Culverts 11 5.1.6 Benches 12 5.1.7 Guards 12 5.1.8 Trail Posts 12 5.2 Next Engineering Steps 12 6 TRAIL MAINTENANCE 12 BC-4Mile Trail Layout Report -ii- APPENDIX A – AIRPHOTO MAP OF TRAIL 13 APPENDIX B – SURVEY MAP OF TRAIL 13 APPENDIX C – ENGINEERED PLAN, PROFILE & CROSS SECTIONS 13 Acknowledgement A number of individuals contributed time and knowledge to this initial stage of locating the proposed trail and Frontier Resource Management Ltd is very grateful for this help. -

Asian Highway Handbook United Nations
ECONOMIC AND SOCIAL COMMISSION FOR ASIA AND THE PACIFIC ASIAN HIGHWAY HANDBOOK UNITED NATIONS New York, 2003 ST/ESCAP/2303 The Asian Highway Handbook was prepared under the direction of the Transport and Tourism Division of the United Nations Economic and Social Commission for Asia and the Pacific. The team of staff members of the Transport and Tourism Division who prepared the Handbook comprised: Fuyo Jenny Yamamoto, Tetsuo Miyairi, Madan B. Regmi, John R. Moon and Barry Cable. Inputs for the tourism- related parts were provided by an external consultant: Imtiaz Muqbil. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has been issued without formal editing. CONTENTS I. INTRODUCTION TO THE ASIAN HIGHWAY………………. 1 1. Concept of the Asian Highway Network……………………………… 1 2. Identifying the Network………………………………………………. 2 3. Current status of the Asian Highway………………………………….. 3 4. Formalization of the Asian Highway Network……………………….. 7 5. Promotion of the Asian Highway……………………………………... 9 6. A Vision of the Future………………………………………………… 10 II. ASIAN HIGHWAY ROUTES IN MEMBER COUNTRIES…... 16 1. Afghanistan……………………………………………………………. 16 2. Armenia……………………………………………………………….. 19 3. Azerbaijan……………………………………………………………... 21 4. Bangladesh……………………………………………………………. 23 5. Bhutan…………………………………………………………………. 27 6. Cambodia……………………………………………………………… 29 7. China…………………………………………………………………... 32 8. Democratic People’s Republic of Korea……………………………… 36 9. Georgia………………………………………………………………... 38 10. India…………………………………………………………………… 41 11. Indonesia………………………………………………………………. 45 12. Islamic Republic of Iran………………………………………………. 49 13 Japan………………………………………………………………….. -

Greenhouse Or Windowsill Growing
Volume 57 Illustrated with more than 150 photographs and drawings- Dwarfed Fr'uit Trees 9y HAROLD BRADFORD TUKEY. In this authoritative guide the author identifies the important dwarfing rootstocks and describes their propagation and growth in the nursery, He discusses the selection and spacing of trees, bracing and trellising, and fruit trees grown under glass, as bonsai and as ornamentals. He also covers tree structure and physiology, modern scientific theories of dwarfing, soils, fertilizers , pollination, costs, yields, and the locations where dwarfed fruit trees are most likely to succeed. Originally published in 1964 and now reissued , this book is unique in its field. 150 black-and-white photographs, 7V2 x 10. Society member's price: $26.55 (Regularly $29.50). "Highly recommended for those interested in maintaining ornamental trees and shrubs." -UbraryJourna/ Insects That Feed on TliAT FEED ON INSECTS AND SHRUBS ~traled An l/Ius Trees and Shrubs By WARREN T. JOHNSON and HOWARD H, LYON. "A beautiful book. It is of high quality stylistically-of folio size, with good paper, of excellent design, and with every other page being a ~;A:";~~: full-color plate .. .. There is no question about the authority and competence of Johnson and Lyon _:.::~:._ _ or of the scientific validity of the book." -Choice. 212 color plates, 9 x 12. Society member}'s price: $31 .50 through June 30, 1978; $34.65 thereafter (Regularly $35.00; $38.50 after June 30 . Of related interest- - ----_._-------- --- __ ----J AGrowth Chamber Manual Environmental Control for Plants Edited by ROBEIH W. LANGHANS. Focusing primarily how to provide precise control, and how to keep a chamber on sophisticated growth chambers used for experimental running at optimum conditions. -

Identification of Rfk-1, a Meiotic Driver Undergoing RNA Editing in Neurospora
HIGHLIGHTED ARTICLE | INVESTIGATION Identification of rfk-1, a Meiotic Driver Undergoing RNA Editing in Neurospora Nicholas A. Rhoades,* Austin M. Harvey,*,1 Dilini A. Samarajeewa,*,1 Jesper Svedberg,† Aykhan Yusifov,* Anna Abusharekh,* Pennapa Manitchotpisit,* Daren W. Brown,‡ Kevin J. Sharp,* David G. Rehard,§,** Joshua Peters,* Xavier Ostolaza-Maldonado,* Jackson Stephenson,* Patrick K. T. Shiu,§ Hanna Johannesson,† and Thomas M. Hammond*,2 *School of Biological Sciences, Illinois State University, Normal, Illinois 61790, †Department of Organismal Biology, Uppsala University, Norbyvägen 18D, 752 36 Uppsala, Sweden, ‡Mycotoxin Prevention and Applied Microbiology, National Center for Agricultural Utilization Research, U.S. Department of Agriculture, Agricultural Research Service, Peoria, Illinois 61604, §Division of Biological Sciences, University of Missouri, Columbia, Missouri 65211, and **Department of Biology, University of Iowa, Iowa 52242 ABSTRACT Sk-2 is a meiotic drive element that was discovered in wild populations of Neurospora fungi over 40 years ago. While early studies quickly determined that Sk-2 transmits itself through sexual reproduction in a biased manner via spore killing, the genetic factors responsible for this phenomenon have remained mostly unknown. Here, we identify and characterize rfk-1, a gene required for Sk-2-based spore killing. The rfk-1 gene contains four exons, three introns, and two stop codons, the first of which undergoes RNA editing to a tryptophan codon during sexual development. Translation of an unedited rfk-1 transcript in vegetative tissue is expected to produce a 102-amino acid protein, whereas translation of an edited rfk-1 transcript in sexual tissue is expected to produce a protein with 130 amino acids. These findings indicate that unedited and edited rfk-1 transcripts exist and that these transcripts could have different roles with respect to the mechanism of meiotic drive by spore killing. -

Chapter A-10 Road Network Development Plan
Final Report The Study on the Road Network Development in the Kingdom of Cambodia October 2006 CHAPTER A-10 ROAD NETWORK DEVELOPMENT PLAN 10.1 Road Development Principle As identified in the existing road condition survey, road network system in Cambodia has sufficient coverage from the perspectives of road density and network, however, many of important roads are not functioning well mainly due to poor surface condition as well as narrow and poor temporary bridges with limited loading capacity. Based on the existing road condition stated above, the Study team establishes the following road development principle; (1) Improvement of Existing Road Network * Use existing road network as much as possible * Improvement of existing road network - Pavement up-grade for 1-Digit and 2-Digit roads - Maintenance for 3-Digit and rural roads (2) Strengthening of Road Network and Capacity * 4 lane widening, Ring Road and Bypass (3) Reinforcement of Road Network * Provision of alternative routes 10.2 Target of Road Network Development Based on the above road development principle as well as the 5 strategies developed in the previous chapter, the following target of road network development was established: Strategy 1: Multi Growth Pole Development Target: (1) Widening and upgrading of 1-Digit road (2) Construction of Bypass around major cities (3) Reinforcement of road network around Phnom Penh by Ring Road Strategy 2: National Integration Target: (1) Improvement of accessibility to provincial capital (2) Reinforcement of main 2-Digit roads Strategy -

Pin Information for the Intel® Agilex™ AGFA022 Device
Pin Information for the Intel® Agilex™ AGFA022 Device Version: 2021-07-07 Status: Final TYPE BANK R24C Package R25A Package R31C Package Transceiver I/O 10A - 68 - Transceiver I/O 10C - 68 - Transceiver I/O 12A - - 84 Transceiver I/O 12C 84 - 84 Transceiver I/O 13A 84 - 84 Transceiver I/O 13C - - 84 GPIO 2C 96 96 96 GPIO 2D 96 - 96 GPIO 2E 72 96 72 GPIO 2F 96 96 96 GPIO 3A 96 48 96 GPIO 3B 96 - 84 GPIO 3C 96 96 84 GPIO 3D 96 96 96 GPIO 3F - 96 - Transceiver I/O 9A - 114 - SDM I/O SDM 29 29 29 i. Total LVDS channels per bank supporting SERDES Non-DPA and DPA mode is equivalent to (LVDS I/O per bank)/2, inclusive of clock pair. Please refer to Dedicated Tx/Rx Channel column in the pin-out table for the channel availability. ii. Total LVDS channels supporting SERDES Soft-CDR mode is 12 pairs per bank. Please refer to Soft CDR column in the pin out table for the channel availability. PT- AGFA022 Copyright © 2021 Intel Corp IO Resource Count Page 1 of 117 Pin Information for the Intel® Agilex™ AGFA022 Device Version: 2021-07-07 Status: Final Bank Number Index within I/O Bank VREF Pin Name/Function Optional Function(s) Configuration Function Dedicated Tx/Rx Channel SERDES Soft CDR Support R24C DQS for X4 DQS for X8/X9 DQS for X16/X18 DQS for X32/X36 SDM TDO CC49 SDM TMS CE43 SDM TCK CF44 SDM TDI CC43 SDM OSC_CLK_1 CB42 SDM SDM_IO0 PWRMGT_SCL,PWRMGT_ALERT CG47 SDM SDM_IO1 AVSTx8_DATA2,AS_DATA1 CA45 SDM SDM_IO5 AS_nCSO0,MSEL0 CC45 SDM SDM_IO3 AVSTx8_DATA3,AS_DATA2 CF46 SDM nCONFIG CC47 SDM SDM_IO4 AVSTx8_DATA1,AS_DATA0 CG45 SDM SDM_IO2 AVSTx8_DATA0,AS_CLK