Transfer from Cox17 to Sco1 Is Coupled to Electron Transfer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COX17 (NM 005694) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC210756 COX17 (NM_005694) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: COX17 (NM_005694) Human Tagged ORF Clone Tag: Myc-DDK Symbol: COX17 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >RC210756 representing NM_005694 Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGCCGGGTCTGGTTGACTCAAACCCTGCCCCGCCTGAGTCTCAGGAGAAGAAGCCGCTGAAGCCCTGCT GCGCTTGCCCGGAGACCAAGAAGGCGCGCGATGCGTGTATCATCGAGAAAGGAGAAGAACACTGTGGACA TCTAATTGAGGCCCACAAGGAATGCATGAGAGCCCTAGGATTTAAAATA ACGCGTACGCGGCCGCTCGAGCAGAAACTCATCTCAGAAGAGGATCTGGCAGCAAATGATATCCTGGATT ACAAGGATGACGACGATAAGGTTTAA Protein Sequence: >RC210756 representing NM_005694 Red=Cloning site Green=Tags(s) MPGLVDSNPAPPESQEKKPLKPCCACPETKKARDACIIEKGEEHCGHLIEAHKECMRALGFKI TRTRPLEQKLISEEDLAANDILDYKDDDDKV Chromatograms: https://cdn.origene.com/chromatograms/mk8114_f02.zip Restriction Sites: SgfI-MluI This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 4 COX17 (NM_005694) Human Tagged ORF Clone – RC210756 Cloning Scheme: Plasmid Map: ACCN: NM_005694 ORF Size: 189 bp This product is -
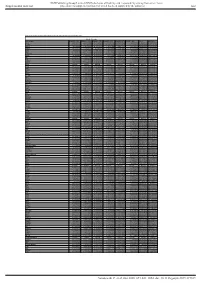
Supl Table 1 for Pdf.Xlsx
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Table S1. Proteomic analysis of liver tissues from 4-months old control and LPTENKO mice. Soluble Fraction Gene names LOG2FC CTL1 LOG2FC CTL2 LOG2FC CTL3 LOG2FC KO1 LOG2FC KO2 LOG2FC KO3 t-test adj Pvalue Aass 0.081686519 -0.097912098 0.016225579 -1.135271836 -0.860535624 -1.263037541 0.0011157 1.206072068 Acsm5 -0.125220746 0.071090866 0.054129881 -0.780692107 -0.882155692 -1.158378647 0.00189031 1.021713016 Gstm2 -0.12707966 0.572554941 -0.445475281 1.952813994 1.856518122 1.561376671 0.00517664 1.865316016 Gstm1 -0.029147714 0.298593425 -0.26944571 0.983159098 0.872028945 0.786125509 0.00721356 1.949464926 Fasn 0.08403202 -0.214149498 0.130117478 1.052480559 0.779734519 1.229308218 0.00383637 0.829422353 Upb1 -0.112438784 -0.137014769 0.249453553 -1.297732445 -1.181999331 -1.495303666 0.00102373 0.184442034 Selenbp2 0.266508271 0.084791964 -0.351300235 -2.040647809 -2.608780781 -2.039865739 0.00107121 0.165425127 Thrsp -0.15001075 0.177999342 -0.027988592 1.54283307 1.603048661 0.927443822 0.00453549 0.612858263 Hyi 0.142635733 -0.183013091 0.040377359 1.325929636 1.119934412 1.181313897 0.00044587 0.053553518 Eci1 -0.119041811 -0.014846366 0.133888177 -0.599970385 -0.555547972 -1.191875541 0.02292305 2.477981171 Aldh1a7 -0.095682449 -0.017781922 0.113464372 0.653424862 0.931724091 1.110750381 0.00356922 0.350756574 Pebp1 -0.06292058 -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Mouse Anti-Human COX17 Monoclonal Antibody, Clone 5H3 (CABT-B10023) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Mouse anti-Human COX17 monoclonal antibody, clone 5H3 (CABT-B10023) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Immunogen COX17 (NP_005685,2a.a. ~ 63 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Isotype IgG2b Source/Host Mouse Species Reactivity Human Clone 5H3 Conjugate Unconjugated Applications WB, IHC, sELISA, ELISA Sequence Similarities MPGLVDSNPAPPESQEKKPLKPCCACPETKKARDACIIEKGEEHCGHLIEAHKECMRALGFKI Format Liquid Buffer In 1x PBS, pH 7.2 Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. BACKGROUND Introduction Cytochrome c oxidase (COX), the terminal component of the mitochondrial respiratory chain, catalyzes the electron transfer from reduced cytochrome c to oxygen. This component is a heteromeric complex consisting of 3 catalytic subunits encoded by mitochondrial genes and multiple structural subunits encoded by nuclear genes. The mitochondrially-encoded subunits function in electron transfer, and the nuclear-encoded subunits may function in the regulation and assembly of the complex. This nuclear gene encodes a protein which is not a structural subunit, but may be involved in the recruitment of copper to mitochondria for incorporation into the COX apoenzyme. This protein shares 92% amino acid sequence identity with mouse and rat Cox17 proteins. This gene is no longer considered to be a candidate gene for COX deficiency. A pseudogene COX17P has been found on chromosome 13. [provided by RefSeq, Jul 2008] 45-1 Ramsey -

Genome-Wide Identification of Mrnas Associated with the Protein SMN Whose Depletion Decreases Their Axonal Localization
Downloaded from rnajournal.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Genome-wide identification of mRNAs associated with the protein SMN whose depletion decreases their axonal localization FLORENCE RAGE,1,2,3 NAWAL BOULISFANE,1,2,3 KHALIL RIHAN,1,2,3 HENRY NEEL,1,2,3,4 THIERRY GOSTAN,1,2,3 EDOUARD BERTRAND,1,2,3 RÉMY BORDONNÉ,1,2,3 and JOHANN SORET1,2,3,5 1Institut de Génétique Moléculaire de Montpellier UMR 5535, 34293 Montpellier Cedex 5, France 2Université Montpellier 2, 34095 Montpellier Cedex 5, France 3Université Montpellier 1, 34967 Montpellier Cedex 2, France ABSTRACT Spinal muscular atrophy is a neuromuscular disease resulting from mutations in the SMN1 gene, which encodes the survival motor neuron (SMN) protein. SMN is part of a large complex that is essential for the biogenesis of spliceosomal small nuclear RNPs. SMN also colocalizes with mRNAs in granules that are actively transported in neuronal processes, supporting the hypothesis that SMN is involved in axonal trafficking of mRNPs. Here, we have performed a genome-wide analysis of RNAs present in complexes containing the SMN protein and identified more than 200 mRNAs associated with SMN in differentiated NSC-34 motor neuron-like cells. Remarkably, ∼30% are described to localize in axons of different neuron types. In situ hybridization and immuno-fluorescence experiments performed on several candidates indicate that these mRNAs colocalize with the SMN protein in neurites and axons of differentiated NSC-34 cells. Moreover, they localize in cell processes in an SMN-dependent manner. Thus, low SMN levels might result in localization deficiencies of mRNAs required for axonogenesis. -

Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease
cells Review Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease Stephanie Gladyck 1, Siddhesh Aras 1,2, Maik Hüttemann 1 and Lawrence I. Grossman 1,2,* 1 Center for Molecular Medicine and Genetics, Wayne State University School of Medicine, Detroit, MI 48201, USA; [email protected] (S.G.); [email protected] (S.A.); [email protected] (M.H.) 2 Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland and Detroit, MI 48201, USA * Correspondence: [email protected] Abstract: Oxidative phosphorylation is a tightly regulated process in mammals that takes place in and across the inner mitochondrial membrane and consists of the electron transport chain and ATP synthase. Complex IV, or cytochrome c oxidase (COX), is the terminal enzyme of the electron transport chain, responsible for accepting electrons from cytochrome c, pumping protons to contribute to the gradient utilized by ATP synthase to produce ATP, and reducing oxygen to water. As such, COX is tightly regulated through numerous mechanisms including protein–protein interactions. The twin CX9C family of proteins has recently been shown to be involved in COX regulation by assisting with complex assembly, biogenesis, and activity. The twin CX9C motif allows for the import of these proteins into the intermembrane space of the mitochondria using the redox import machinery of Mia40/CHCHD4. Studies have shown that knockdown of the proteins discussed in this review results in decreased or completely deficient aerobic respiration in experimental models ranging from yeast to human cells, as the proteins are conserved across species. -

Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces Cerevisiae
life Review Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces cerevisiae Leticia V. R. Franco 1,2,* , Luca Bremner 1 and Mario H. Barros 2 1 Department of Biological Sciences, Columbia University, New York, NY 10027, USA; [email protected] 2 Department of Microbiology,Institute of Biomedical Sciences, Universidade de Sao Paulo, Sao Paulo 05508-900, Brazil; [email protected] * Correspondence: [email protected] Received: 27 October 2020; Accepted: 19 November 2020; Published: 23 November 2020 Abstract: The ease with which the unicellular yeast Saccharomyces cerevisiae can be manipulated genetically and biochemically has established this organism as a good model for the study of human mitochondrial diseases. The combined use of biochemical and molecular genetic tools has been instrumental in elucidating the functions of numerous yeast nuclear gene products with human homologs that affect a large number of metabolic and biological processes, including those housed in mitochondria. These include structural and catalytic subunits of enzymes and protein factors that impinge on the biogenesis of the respiratory chain. This article will review what is currently known about the genetics and clinical phenotypes of mitochondrial diseases of the respiratory chain and ATP synthase, with special emphasis on the contribution of information gained from pet mutants with mutations in nuclear genes that impair mitochondrial respiration. Our intent is to provide the yeast mitochondrial specialist with basic knowledge of human mitochondrial pathologies and the human specialist with information on how genes that directly and indirectly affect respiration were identified and characterized in yeast. Keywords: mitochondrial diseases; respiratory chain; yeast; Saccharomyces cerevisiae; pet mutants 1. -

A 5 Cytosine Binding Pocket in Puf3p Specifies Regulation Of
A5 cytosine binding pocket in Puf3p specifies regulation of mitochondrial mRNAs Deyu Zhua,1, Craig R. Stumpfb,1, Joseph M. Krahna, Marvin Wickensb,2, and Traci M. Tanaka Halla,2 aLaboratory of Structural Biology, National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709; and bDepartment of Biochemistry, University of Wisconsin-Madison, Madison, WI 53706 Edited by Keith R. Yamamoto, University of California, San Francisco, CA, and approved October 2, 2009 (received for review November 26, 2008) A single regulatory protein can control the fate of many mRNAs biological importance of the RNA base at the Ϫ2 position for with related functions. The Puf3 protein of Saccharomyces cerevi- regulation in vivo. siae is exemplary, as it binds and regulates more than 100 mRNAs that encode proteins with mitochondrial function. Here we eluci- Results date the structural basis of that specificity. To do so, we explore the General Puf3p Architecture and the Core Region. Crystal structures crystal structures of Puf3p complexes with 2 cognate RNAs. The key of the RNA-binding domain of Puf3p in complex with the determinant of Puf3p specificity is an unusual interaction between COX17 mRNA sequences for binding site A, CUUGUAUAUA, a distinctive pocket of the protein with an RNA base outside the and site B, CCUGUAAAUA (Fig. 1A), were determined at a ‘‘core’’ PUF-binding site. That interaction dramatically affects bind- resolution of 3.2 and 2.5 Å, respectively. Puf3p interacts with the ing affinity in vitro and is required for regulation in vivo. The Puf3p 8 bases of COX17 mRNA starting from the conserved UGUA structures, combined with those of Puf4p in the same organism, sequence much as human Pumilio1 (PUM1) binds to the equiv- illuminate the structural basis of natural PUF-RNA networks. -

Mitochondrial Copper Homeostasis in Mammalian Cells
Mitochondrial copper homeostasis in mammalian cells Dissertation zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Fakultät Mathematik und Naturwissenschaften der Technischen Universität Dresden von Corina Oswald (Diplom-Biochemikerin) geboren am 10.04.1981 in Dohna, Deutschland Gutachter: Prof. Dr. Gerhard Rödel Prof. Dr. Alexander Storch Eingereicht am 30. April 2010 Verteidigt am 13. August 2010 ACKNOWLEDGEMENTS I sincerely thank my supervisor Prof. Dr. Gerhard Rödel for giving me the opportunity to do my PhD in his group and to join the Dresden International Graduate School for Biomedicine and Bioengineering (DIGS-BB). He introduced me to the world of mitochondria, supported and provided me with all resources and comprehension necessary to conduct my research. I thank Dr. Udo Krause-Buchholz for his scientific advice and for helping writing the paper by giving constructive comments on the manuscript. I honestly thank my TAC members Dr. Frank Buchholz and Prof. Dr. Alexander Storch for their interest in this work, for guiding me scientifically, and for stimulating discussions in the TAC meeting. Especially, Dr. Frank Bucholz for giving insightful suggestions as RNAi specialist, and Prof. Dr. Alexander Storch for acting as reviewer of this thesis. The dSTORM images would not have been possible without the very friendly collaboration with Prof. Dr. Markus Sauer and Sebastian van de Linde, Institute for Applied Laser Physics and Laser Spectroscopy of the University of Bielefeld. Thank you! I am furthermore grateful to all former and present lab members for the friendly working atmosphere, for fruitful discussions, for providing advice and assistance in many situations. -

Original Article Stable COX17 Downregulation Leads To
Original Article Stable COX17 Downregulation Leads to Alterations in Mitochondrial Ultrastructure, Decreased Copper Content and Impaired Cytochrome c Oxidase Biogenesis in HEK293 Cells (Cox17 protein / copper chaperone / cytochrome c oxidase assembly / mitochondrial ultrastructure) M. VANIŠOVÁ, D. BURSKÁ, J. KŘÍŽOVÁ, T. DAŇHELOVSKÁ, Ž. DOSOUDILOVÁ, J. ZEMAN, L. STIBŮREK, H. HANSÍKOVÁ Laboratory for Study of Mitochondrial Disorders, Department of Paediatrics and Adolescent Medicine, First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic Abstract. Cox17 is an assembly factor that par- COX17 shRNA-downregulated HEK293 cell lines ticipates in early cytochrome c oxidase (COX, CcO) that have less than 10 % of the residual Cox17 pro- assembly stages. Cox17 shuttles copper ions from the tein level. Cox17-depleted cell lines exhibited decreased cytosol to the mitochondria and, together with Sco1 intramitochondrial copper content, decreased CcO and Sco2, provides copper ions to the Cox1 and Cox2 subunit levels (Cox1, Cox4 and Cox5a) and accumu- mitochondrially encoded subunits. In Saccharomyces lation of CcO subcomplexes. Similarly to yeast cells, cerevisiae, Cox17 also modulates mitochondrial mem- mitochondria in Cox17-downregulated HEK293 cell brane architecture due to the interaction of Cox17 lines exhibited ultrastructural changes including with proteins of the MICOS complex (mitochondrial cristae reduction and mitochondrial swelling. Charac- contact site and cristae organizing system). There is terization of the molecular pathogenesis of long-term currently no data regarding the impact of long-term Cox17 deficiency complements our knowledge of the Cox17 deficiency in human cells. Here, we present mitochondrial copper metabolism and assembly of construction and characterization of three stable cytochrome c oxidase in human cells. -

Output Results of CLIME (Clustering by Inferred Models of Evolution)
Output results of CLIME (CLustering by Inferred Models of Evolution) Dataset: Num of genes in input gene set: 81 Total number of genes: 20834 Prediction LLR threshold: 0 The CLIME PDF output two sections: 1) Overview of Evolutionarily Conserved Modules (ECMs) Top panel shows the predefined species tree. Bottom panel shows the partition of input genes into Evolutionary Conserved Modules (ECMs), ordered by ECM strength (shown at right), and separated by horizontal lines. Each row show one gene, where the phylogenetic profile indicates presence (blue) or absence (gray) of homologs in each species (column). Gene symbols are shown at left. Gray color indicates that the gene is a paralog to a higher scoring gene within the same ECM (based on BLASTP E < 1e-3). 2) Details of each ECM and its expansion ECM+ Top panel shows the inferred evolutionary history on the predefined species tree. Branch color shows the gain event (blue) and loss events (red color, with brighter color indicating higher confidence in loss). Branches before the gain or after a loss are shown in gray. Bottom panel shows the input genes that are within the ECM (blue/white rows) as well as all genes in the expanded ECM+ (green/gray rows). The ECM+ includes genes likely to have arisen under the inferred model of evolution relative to a background model, and scored using a log likelihood ratio (LLR). PG indicates "paralog group" and are labeled alphabetically (i.e., A, B). The first gene within each paralog group is shown in black color. All other genes sharing sequence similarity (BLAST E < 1e-3) are assigned to the same PG label and displayed in gray. -

Downloaded Per Proteome Cohort Via the Web- Site Links of Table 1, Also Providing Information on the Deposited Spectral Datasets
www.nature.com/scientificreports OPEN Assessment of a complete and classifed platelet proteome from genome‑wide transcripts of human platelets and megakaryocytes covering platelet functions Jingnan Huang1,2*, Frauke Swieringa1,2,9, Fiorella A. Solari2,9, Isabella Provenzale1, Luigi Grassi3, Ilaria De Simone1, Constance C. F. M. J. Baaten1,4, Rachel Cavill5, Albert Sickmann2,6,7,9, Mattia Frontini3,8,9 & Johan W. M. Heemskerk1,9* Novel platelet and megakaryocyte transcriptome analysis allows prediction of the full or theoretical proteome of a representative human platelet. Here, we integrated the established platelet proteomes from six cohorts of healthy subjects, encompassing 5.2 k proteins, with two novel genome‑wide transcriptomes (57.8 k mRNAs). For 14.8 k protein‑coding transcripts, we assigned the proteins to 21 UniProt‑based classes, based on their preferential intracellular localization and presumed function. This classifed transcriptome‑proteome profle of platelets revealed: (i) Absence of 37.2 k genome‑ wide transcripts. (ii) High quantitative similarity of platelet and megakaryocyte transcriptomes (R = 0.75) for 14.8 k protein‑coding genes, but not for 3.8 k RNA genes or 1.9 k pseudogenes (R = 0.43–0.54), suggesting redistribution of mRNAs upon platelet shedding from megakaryocytes. (iii) Copy numbers of 3.5 k proteins that were restricted in size by the corresponding transcript levels (iv) Near complete coverage of identifed proteins in the relevant transcriptome (log2fpkm > 0.20) except for plasma‑derived secretory proteins, pointing to adhesion and uptake of such proteins. (v) Underrepresentation in the identifed proteome of nuclear‑related, membrane and signaling proteins, as well proteins with low‑level transcripts.