Chapter 18 Nuclear Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 11. Nuclear Chemistry 11.1 Stable and Unstable Nuclides Very Large
11. Nuclear Chemistry Chemical reactions occur as a result of loosing/gaining and sharing electrons in the valance shell which is far away from the atomic nucleus as we described in previous chapters in chemical bonding. In chemical reactions identity of the elements (atomic) and the makeup of the nuclei (mass due to protons and neutrons) is preserved which is reflected in the Law of Conservation of mass. This idea of atomic nucleus is always stable was shattered as Henri Becquerel discovered radioactivity in uranium compound where uranium nuclei changes or undergo nuclear reactions where nuclei of an element is transformed into nuclei of different element(s) while emitting ionization radiation. Marie Curie also began a study of radioactivity in a different form of uranium ore called pitchblende and she discovered the existence of two more highly radioactive new elements radium and polonium formed as the products during the decay of unstable nuclide of uranium-235. Curie measure that the radiation emanated was proportional to the amount (moles or number of nuclides) of radioactive element present, and she proposed that radiation was a property nucleus of an unstable atom. The area of chemistry that focuses on the nuclear changes is called nuclear chemistry. What changes in a nuclide result from the loss of each of the following? a) An alpha particle. b) A gamma ray. c) An electron. d) A neutron. e) A proton. Answer: a), c), d), e) 11.1 Stable and Unstable Nuclides There are stable and unstable radioactive nuclides. Unstable nuclides emit subatomic particles, with alpha −α, beta −β, gamma −γ, proton-p, neutrons-n being the most common. -

Unit 1Moles and Equations
Cambridge University Press 978-1-107-61665-3 – Cambridge International AS and A Level Chemistry Judith Potter Peter Cann Excerpt More information Moles and equations Unit 1 Learning outcomes You should be able to: defi ne and use the terms empirical and molecular formulae and use experimental data defi ne and use relative masses based on the to fi nd them, 12C scale write and construct equations and use them in defi ne and use the mole and Avogadro’s performing mole calculations constant perform mole calculations involving solutions analyse simple mass spectra and use the data in and gases, relative atomic mass calculations This unit gathers together the basic chemistry relative isotopic mass = calculations. You may need to refer to it as you revise mass of one atom of the isotope other topics in later units. (1/12) of the mass of one atom of 12C Relative masses are a ratio of two masses and have 1.01 Relative masses no units. An atom is the smallest part of an element that can exist and still retain the identity of the element. A 1.02 The mole and the molecule is a group of atoms bonded together and the smallest portion of an element or compound that can Avogadro constant exist alone. A simple ion is an atom which has lost or To give an easy measurement of mass, a number of gained one or more electrons. Atoms, molecules and atoms is chosen so that their combined mass is equal to ions are so small that their masses are far too small the relative atomic mass in grams. -

Chromium Isotopes Detection in Their Ores with Minimal Errors
Open Access Annals of Advances in Chemistry Research Article Chromium Isotopes Detection in their Ores with Minimal Errors ISSN 2576-3768 Loai Aljerf1* and Nuha AlMasri2 1Department of Basic Sciences, Faculty of Dental Medicine, Damascus University, Mazzeh Highway, Almazzeh, Damascus, Syria 2Department of Chemistry, Faculty of Medicine, Syrian Private University, Damascus, Syria *Address for Correspondence: Loai Aljerf, De- Abstract partment of Basic Sciences, Faculty of Dental Medicine, Damascus University, Mazzeh High- The industrial production and use of chromium have grown considerably during the past way, Almazzeh, Damascus, Syria, Tel: +963-93 fi ve decades. Abundances of the chromium isotopes in terrestrial samples are identical to 34 46 993; Email: [email protected]; 0.01%. Among the dominant species of chromium, the trivalent form widely occurs in nature in [email protected] chromite ores which is extremely immobilized especially in water bodies. Samples were mixtures Submitted: 21 July 2018 of separated chromium isotopes and the calibration was made with the same species as those Approved: 03 September 2018 used in the measurements. The method had simplifi ed the conversion of the ores to chromyl Published: 04 September 2018 fl uoride since the element could be readily separated as lead chromate from the leaching of Copyright: 2018 Aljerf L, et al. This is an open chromite-sodium peroxide fusions. Isotope assay of chromyl fl uoride under certain conditions access article distributed under the Creative was measured and the measurements of chromium isotopic anomalies ratios and isotope Commons Attribution License, which permits abundance of the chromite ores have been assessed. These provided suffi cient quantitative mass unrestricted use, distribution, and reproduction spectrometric data, which were analyzed to calculate the abundance and the mean atomic mass in any medium, provided the original work is of the questioned isotopes. -

Determination of Picogram Quantities of Vanadium in Calcite And
+ + Anal. Chem. 1996, 68, 371-377 Determination of Picogram Quantities of Vanadium in Calcite and Seawater by Isotope Dilution Inductively Coupled Plasma Mass Spectrometry with Electrothermal Vaporization David W. Hastings* and Steven R. Emerson School of Oceanography, WB-10, University of Washington, Seattle, Washington 98195 Bruce K. Nelson Department of Geological Sciences, AJ-20, University of Washington, Seattle, Washington 98195 We developed a method to measure picogram quantities Recent interest in the geochemistry of vanadium has arisen of vanadium in calcite and seawater by isotope dilution from its unique redox behavior 9,10 and the observation of enriched (ID) inductively coupled plasma mass spectrometry using concentrations in anoxic sediments, black shale, and crude oil.11,12 electrothermal vaporization (ETV) to introduce the sample Because V deposition is redox sensitive, its concentration in into the plasma. A 50V isotope spike enriched to 44 atom seawater is most likely sensitive to the oxidation state of sedi- % was equilibrated with samples, followed by chemical ments. The calcitic shells of foraminifera accurately record purification by cation exchange chromatography. Samples seawater concentrations of V,13 so the V content of the fossil shells were introduced into the ETV unit with a Pd modifier and of these microscopic oceanic organisms potentially can serve as heated to 1000 °C. This quantitatively eliminates the an indicator of changes in past ocean anoxia.14 ClO+ isobaric interference with V at m/z 51 for solutions A typical 5 mg hand-picked sample of these microscopic shells up to 0.5 N HCl. The procedural blank was 0.27 pg of yields only 50-200 pg of vanadium. -
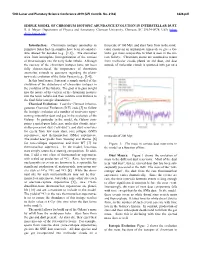
Simple Model of Chromium Isotopic Abundance Evolution in Interstellar Dust
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 3226.pdf SIMPLE MODEL OF CHROMIUM ISOTOPIC ABUNDANCE EVOLUTION IN INTERSTELLAR DUST. B. S. Meyer,. Department of Physics and Astronomy, Clemson University, Clemson, SC 29634-0978, USA (mbra- [email protected]). Introduction: Chromium isotopic anomalies in timescale of 100 Myr, and stars form from in the mole- primitive Solar System samples have been of consider- cular clouds on an appropriate timescale to give a Ga- able interest for decades (e.g., [1,2]). The anomalies lactic gas mass comparable to what is seen in the cur- arise from incomplete homogenization of the carriers rent Galaxy. Chromium atoms are assumed to return of these isotopes into the early Solar nebula. Although from molecular clouds plated on old dust, and dust the carriers of the chromium isotopes have not been outside of molecular clouds is sputtered into gas on a fully characterized, the importance of chromium anomalies extends to questions regarding the plane- tary-scale evolution of the Solar System (e.g., [3,4]). In this brief paper, I present a simple model of the evolution of the abundances of chromium isotopes in the evolution of the Galaxy. The goal is to gain insight into the nature of the carriers of the chromium isotopes into the Solar nebula and their relative contributions to the final Solar isotopic abundances. Chemical Evolution: I use the Clemson Inhomo- geneous Chemical Evolution (ICE) code [5] to follow the isotopic evolution of a number of reservoirs repre- senting interstellar dust and gas in the evolution of the Galaxy. -

Chromium Isotope Behaviour in Natural Waters
UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Chromium Isotope Behaviour in Natural Waters by Heather Jane Goring-Harford Thesis for the degree of Doctor of Philosophy June 2017 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Doctor of Philosophy CHROMIUM ISOTOPE BEHAVIOUR IN NATURAL WATERS By Heather Jane Goring-Harford The isotopes of chromium (Cr) fractionate during terrestrial oxidation reactions that require the presence of oxygen. The main source of Cr to the oceans is via rivers, and thus Cr isotopic signatures (expressed as δ53Cr) preserved in authigenic sediments are increasingly being used to reconstruct the oxygen levels of ancient environments. However, Cr can undergo various reactions in natural waters that may fractionate Cr isotopes. The contribution of these reactions to authigenic sediment δ53Cr values is not well understood, and this limits the interpretation of δ53Cr values measured in ancient archives. This thesis describes the development of a method to accurately and precisely measure Cr isotopic signatures in natural waters with extremely low Cr concentrations, and evaluates the behaviour of Cr isotopes in water samples from a range of environmental settings. Seawater samples from the Atlantic Ocean Oxygen Minimum Zone (OMZ) were analysed to evaluate whether Cr isotopic fractionation was enhanced under depleted oxygen conditions. Results indicate that the Atlantic OMZ is not sufficiently depleted in oxygen to reduce and remove Cr from seawater, although δ53Cr values (1.08 – 1.72‰) were variable due to adsorption of Cr(III) to particles on the shelf, and hydrological mixing. Black Sea seawater samples that had dissolved oxygen concentrations close to zero were enriched in 52Cr, resulting in a δ53Cr that was 0.38‰ lower than the overlying fully oxygenated waters. -

Background and Derivation of ANS-5.4 Standard Fission Product Release Model
NUREG/CR-7003 PNNL-18490 Background and Derivation of ANS-5.4 Standard Fission Product Release Model Office of Nuclear Regulatory Research AVAILABILITY OF REFERENCE MATERIALS IN NRC PUBLICATIONS NRC Reference Material Non-NRC Reference Material As of November 1999, you may electronically access Documents available from public and special technical NUREG-series publications and other NRC records at libraries include all open literature items, such as NRC’s Public Electronic Reading Room at books, journal articles, and transactions, Federal http://www.nrc.gov/reading-rm.html. Register notices, Federal and State legislation, and Publicly released records include, to name a few, congressional reports. Such documents as theses, NUREG-series publications; Federal Register notices; dissertations, foreign reports and translations, and applicant, licensee, and vendor documents and non-NRC conference proceedings may be purchased correspondence; NRC correspondence and internal from their sponsoring organization. memoranda; bulletins and information notices; inspection and investigative reports; licensee event reports; and Commission papers and their attachments. Copies of industry codes and standards used in a substantive manner in the NRC regulatory process are NRC publications in the NUREG series, NRC maintained at— regulations, and Title 10, Energy, in the Code of The NRC Technical Library Federal Regulations may also be purchased from one Two White Flint North of these two sources. 11545 Rockville Pike 1. The Superintendent of Documents Rockville, MD 20852–2738 U.S. Government Printing Office Mail Stop SSOP Washington, DC 20402–0001 These standards are available in the library for Internet: bookstore.gpo.gov reference use by the public. Codes and standards are Telephone: 202-512-1800 usually copyrighted and may be purchased from the Fax: 202-512-2250 originating organization or, if they are American 2. -

The Delimiting/Frontier Lines of the Constituents of Matter∗
The delimiting/frontier lines of the constituents of matter∗ Diógenes Galetti1 and Salomon S. Mizrahi2 1Instituto de Física Teórica, Universidade Estadual Paulista (UNESP), São Paulo, SP, Brasily 2Departamento de Física, CCET, Universidade Federal de São Carlos, São Carlos, SP, Brasilz (Dated: October 23, 2019) Abstract Looking at the chart of nuclides displayed at the URL of the International Atomic Energy Agency (IAEA) [1] – that contains all the known nuclides, the natural and those produced artificially in labs – one verifies the existence of two, not quite regular, delimiting lines between which dwell all the nuclides constituting matter. These lines are established by the highly unstable radionuclides located the most far away from those in the central locus, the valley of stability. Here, making use of the “old” semi-empirical mass formula for stable nuclides together with the energy-time uncertainty relation of quantum mechanics, by a simple calculation we show that the obtained frontier lines, for proton and neutron excesses, present an appreciable agreement with the delimiting lines. For the sake of presenting a somewhat comprehensive panorama of the matter in our Universe and their relation with the frontier lines, we narrate, in brief, what is currently known about the astrophysical nucleogenesis processes. Keywords: chart of nuclides, nucleogenesis, nuclide mass formula, valley/line of stability, energy-time un- certainty relation, nuclide delimiting/frontier lines, drip lines arXiv:1909.07223v2 [nucl-th] 22 Oct 2019 ∗ This manuscript is based on an article that was published in Brazilian portuguese language in the journal Revista Brasileira de Ensino de Física, 2018, 41, e20180160. http://dx.doi.org/10.1590/ 1806-9126-rbef-2018-0160. -

ILPAC Unit S2: Atomic Structure
UNIT INDEPENDENT LEARNING PROJECT FOR ADVANCED CHEMISTRY Periodic Table of the Elements o 2 I He II ill] III IV V VI VIII 4.0 3 4 5 6 7 8 9 10 Li Be B C N 0 F Ne 6.9 9.0 10.8 12.0 14.0 16.0 19.0 20.2 11 12 13 14 15 16 17 18 Na Mg Al Si P S CI Ar 23.0 24.3 27.0 28.1 31.0 32.1 35.5 39.9 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 3! 36 K Ca Sc Ti V Cr Mn Fe Co Ni eu Zn Ga Ge As Se BrlKr 39.1 40.1 45.0 47.9 50.9 52.0 54.9 55.9 58.9 58.7 63.5 65.4 69.7 72.6 74.9 79.0 79 83.8 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 S4 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85.5 87.6 88.9 91.2 92.9 95.9 99.0 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.91 ' 3 ' . 3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba La 4 Hf Ta W Re Os If Pt Au Hg Tl Pb Bi Po AtlRn 132.9 137.3 138.9 178.5 181.0 183.9 186.2 190.2 192.2 195.1 197.0 200.6 204.4 207.2 209.0 210.0 210.01222.0 87 88 89 Fr Ra Ac~ 223.0 226.0 227.0 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 "--- Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lw 232.0 231.0 238.1 (237) 239.1 (243) (241) (247) (251 ) (254) (253) (256) ( 254) (257) A value in brackets denotes the mass number of the most stable isotope. -

45024017.Pdf
Ain Shams University Faculty of Science Radiochemical Study on the Separation of Chromium-51 from the Irradiated Target by Using Commercial and/or Synthesized Ion Exchanger A thesis Submitted by Mohamed Ismail Mohamed Mohamed Aydia (M. Sc. Inorganic and Physical Chemistry 2007) Chemist\ Radioisotopes Production Facility Egyptian Second Research Reactor Atomic Energy Authority For the Degree of Doctor of Philosophy in Science (Chemistry). Department of Chemistry Faculty of Science Ain Shams University (2012) Ain Shams University Faculty of Science Approval Sheet The Thesis entitled Radiochemical Study on the Separation of Chromium-51 from the Irradiated Target by Using Commercial and/or Synthesized Ion Exchanger Submitted by/ Mohamed Ismail Mohamed Aydia (M. Sc. Inorganic and Physical Chemistry 2007) For the Degree of Doctor of Philosophy in Science (Chemistry). This thesis has been approved by supervisor committee SUPERVISORS • Prof. Dr. M.S. Abdel-Mottaleb ….……………… Professor of Inorganic Chemistry Faculty of Science-Ain Shams University • Prof. Dr. Salah Soliman ….……………… Ex-Chairman of Hot Labs Center Supervisor of Radioisotopes Production Facility Atomic Energy Authority • Prof. Dr. A. A. El-Mohty ….……………… Professor of Radiochemistry Radioactive Isotopes and Generator Dep. Atomic Energy Authority • Prof. K.M. El-Azony ….……………… Associate Professor of Radiochemistry Radioactive Isotopes and Generator Dep. Atomic Energy Authority. Prof. Dr. Maged Shafik Antonious Head of Chemistry Department Faculty of Science - Ain Shams University CHAPTER (I) INTRODUCTION CHAPTER (II) EXPERIMENTAL CHAPTER (III) RESULTS AND DISCUSSION REFERENCES ] SUMMARY ARABIC SUMMARY ACKNOWLEDGEMENT I would like to express my gratitude to Prof. Dr. Mohamed Sabry Abdel-Mottaleb, Professor of Inorganic Chemistry, Faculty of Science, Ain Shams University for his supervision and his sponsorship for this work. -

Manual for Reactor Produced Radioisotopes
IAEA-TECDOC-1340 Manual for reactor produced radioisotopes January 2003 The originating Section of this publication in the IAEA was: Industrial Applications and Chemistry Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria MANUAL FOR REACTOR PRODUCED RADIOISOTOPES IAEA, VIENNA, 2003 IAEA-TECDOC-1340 ISBN 92–0–101103–2 ISSN 1011–4289 © IAEA, 2003 Printed by the IAEA in Austria January 2003 FOREWORD Radioisotopes find extensive applications in several fields including medicine, industry, agriculture and research. Radioisotope production to service different sectors of economic significance constitutes an important ongoing activity of many national nuclear programmes. Radioisotopes, formed by nuclear reactions on targets in a reactor or cyclotron, require further processing in almost all cases to obtain them in a form suitable for use. Specifications for final products and testing procedures for ensuring quality are also an essential part of a radioisotope production programme. The International Atomic Energy Agency (IAEA) has compiled and published such information before for the benefit of laboratories of Member States. The first compilation, entitled Manual of Radioisotope Production, was published in 1966 (Technical Reports Series No. 63). A more elaborate and comprehensive compilation, entitled Radioisotope Production and Quality Control, was published in 1971 (Technical Reports Series No. 128). Both served as useful reference sources for scientists working in radioisotope production worldwide. -

USER's GUIDE: RADIONUCLIDE CARCINOGENICITY Introduction
USER'S GUIDE: RADIONUCLIDE CARCINOGENICITY Introduction EPA classifies all radionuclides as Group A carcinogens. The Radionuclide Table on this website, formerly HEAST Table 4, lists ingestion, inhalation and external exposure cancer slope factors (risk coefficients for total cancer morbidity) for radionuclides in conventional units of picocuries (pCi). (7) Ingestion and inhalation slope factors are central estimates in a linear model of the age-averaged, lifetime attributable radiation cancer incidence (fatal and nonfatal cancer) risk per unit of activity inhaled or ingested, expressed as risk/pCi. External exposure slope factors are central estimates of lifetime attributable radiation cancer incidence risk for each year of exposure to external radiation from photon-emitting radionuclides distributed uniformly in a thick layer of soil and are expressed as risk/yr per pCi/gram soil. When combined with site-specific media concentration data and appropriate exposure assumptions (8), slope factors can be used to estimate lifetime cancer risks to members of the general population due to radionuclide exposures. Intended Users and Applications HEAST users include individuals from the EPA, other Federal agencies, States and contractors who are responsible for the identification, characterization and remediation of sites contaminated with radioactive materials. Radionuclide slope factors are calculated by EPA's Office of Radiation and Indoor Air (ORIA) to assist HEAST users with risk-related evaluations and decision-making at various stages of the remediation process. During site assessment, for example, slope factors are used in EPA's Hazard Ranking System (HRS) to assign toxicity factor values to radionuclides to calculate site scores. During the remedial investigation and feasibility study (RI/FS), slope factors are used to determine baseline site risk, to develop preliminary remediation goals, and to evaluate cleanup alternatives.