The Spatial Configuration of Greenspace Affects Semi-Aquatic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EMYDIDAE P Catalogue of American Amphibians and Reptiles
REPTILIA: TESTUDINES: EMYDIDAE P Catalogue of American Amphibians and Reptiles. Pseudemysj7oridana: Baur, 1893:223 (part). Pseudemys texana: Brimley, 1907:77 (part). Seidel, M.E. and M.J. Dreslik. 1996. Pseudemys concinna. Chrysemysfloridana: Di tmars, 1907:37 (part). Chrysemys texana: Hurter and Strecker, 1909:21 (part). Pseudemys concinna (LeConte) Pseudemys vioscana Brimley, 1928:66. Type-locality, "Lake River Cooter Des Allemands [St. John the Baptist Parrish], La." Holo- type, National Museum of Natural History (USNM) 79632, Testudo concinna Le Conte, 1830: 106. Type-locality, "... rivers dry adult male collected April 1927 by Percy Viosca Jr. of Georgia and Carolina, where the beds are rocky," not (examined by authors). "below Augusta on the Savannah, or Columbia on the Pseudemys elonae Brimley, 1928:67. Type-locality, "... pond Congaree," restricted to "vicinity of Columbia, South Caro- in Guilford County, North Carolina, not far from Elon lina" by Schmidt (1953: 101). Holotype, undesignated, see College, in the Cape Fear drainage ..." Holotype, USNM Comment. 79631, dry adult male collected October 1927 by D.W. Tesrudofloridana Le Conte, 1830: 100 (part). Type-locality, "... Rumbold and F.J. Hall (examined by authors). St. John's river of East Florida ..." Holotype, undesignated, see Comment. Emys (Tesrudo) concinna: Bonaparte, 1831 :355. Terrapene concinna: Bonaparte, 183 1 :370. Emys annulifera Gray, 183 1:32. Qpe-locality, not given, des- ignated as "Columbia [Richland County], South Carolina" by Schmidt (1953: 101). Holotype, undesignated, but Boulenger (1889:84) listed the probable type as a young preserved specimen in the British Museum of Natural His- tory (BMNH) from "North America." Clemmys concinna: Fitzinger, 1835: 124. -

Get to Know Your Neighborhood Turtles! We All Love Turtles
Originally published in the October and November 2017 Woodlake Life on the Lake Newsletter Get to know your neighborhood turtles! We all love turtles. Some of us have pet turtles and some of us are big fans of Ninja turtles. But we can do a little bit more to secure the future of these fascinating creatures. This article introduces the turtles in Woodlake and what can we do to protect them. I was driving on the Woodlake Village Parkway with my daughter, when we spotted a box turtle crossing the road. We changed the lane to avoid hitting the turtle, and turned around hoping to help the turtle cross the road. After 10 seconds, we were so sad to find the crushed turtle on the middle of the road. That was a very upsetting moment for both me and my four year old. May be the driver of the big truck did not see it…or may be the driver was too distracted to avoid it. Vehicle collisions are one of the major causes of turtle mortality. But if we were willing to stop or change the lane, we could have saved many of those turtles! Turtles are fascinating creatures for many reasons! Some ancient civilizations considered turtles as sacred animals and they believed that our world is supported by a giant “cosmic turtle”. Turtles are one of the oldest vertebrate groups on the planet earth, roaming around for about 220 million years, since before the time of dinosaurs. At the same time, turtles have a really long lifespan and scientists believe that many of them can easily surpass 100 years. -

Indigenous and Established Herpetofauna of Northwest Louisiana
Indigenous and Established Herpetofauna of Northwest Louisiana Non-venomous Snakes (25 Species) For more info: Buttermilk Racer Coluber constrictor anthicus 318-773-9393 Eastern Coachwhip Coluber flagellum flagellum www.learnaboutcritters.org Prairie Kingsnake Lampropeltis calligaster [email protected] Speckled Kingsnake Lampropeltis holbrooki www.facebook.com/learnaboutcritters Western Milksnake Lampropeltis gentilis Northern Rough Greensnake Opheodrys aestivus aestivus Alligator (1 species) Western Ratsnake Pantherophis obsoletus American Alligator Alligator mississippiensis Slowinski’s Cornsnake † Pantherophis slowinskii Flat-headed Snake † Tantilla gracilis Western Wormsnake † Carphophis vermis Lizards (10 Species) Mississippi Ring-necked Snake Diadophis punctatus stictogenys Western Mudsnake Farancia abacura reinwardtii Northern Green Anole Anolis carolinensis Eastern Hog-nosed Snake Heterodon platirhinos Prairie Lizard Sceloporus consobrinus Mississippi Green Watersnake Nerodia cyclopion Southern Coal Skink † Plestiodon anthracinus pluvialis Plain-bellied Watersnake Nerodia erythrogaster Common Five-lined Skink Plestiodon fasciatus Broad-banded Watersnake Nerodia fasciata confluens Broad-headed Skink Plestiodon laticeps Graham's Crayfish Snake Regina grahamii Southern Prairie Skink † Plestiodon septentrionalis obtusirostris Gulf Swampsnake Liodytes rigida sinicola Little Brown Skink Scincella lateralis Dekay’s Brownsnake Storeria dekayi Eastern Six-lined Racerunner Aspidoscelis sexlineata sexlineata Red-bellied Snake -

Standard Common and Current Scientific Names for North American Amphibians, Turtles, Reptiles & Crocodilians
STANDARD COMMON AND CURRENT SCIENTIFIC NAMES FOR NORTH AMERICAN AMPHIBIANS, TURTLES, REPTILES & CROCODILIANS Sixth Edition Joseph T. Collins TraVis W. TAGGart The Center for North American Herpetology THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY www.cnah.org Joseph T. Collins, Director The Center for North American Herpetology 1502 Medinah Circle Lawrence, Kansas 66047 (785) 393-4757 Single copies of this publication are available gratis from The Center for North American Herpetology, 1502 Medinah Circle, Lawrence, Kansas 66047 USA; within the United States and Canada, please send a self-addressed 7x10-inch manila envelope with sufficient U.S. first class postage affixed for four ounces. Individuals outside the United States and Canada should contact CNAH via email before requesting a copy. A list of previous editions of this title is printed on the inside back cover. THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY BO A RD OF DIRE ct ORS Joseph T. Collins Suzanne L. Collins Kansas Biological Survey The Center for The University of Kansas North American Herpetology 2021 Constant Avenue 1502 Medinah Circle Lawrence, Kansas 66047 Lawrence, Kansas 66047 Kelly J. Irwin James L. Knight Arkansas Game & Fish South Carolina Commission State Museum 915 East Sevier Street P. O. Box 100107 Benton, Arkansas 72015 Columbia, South Carolina 29202 Walter E. Meshaka, Jr. Robert Powell Section of Zoology Department of Biology State Museum of Pennsylvania Avila University 300 North Street 11901 Wornall Road Harrisburg, Pennsylvania 17120 Kansas City, Missouri 64145 Travis W. Taggart Sternberg Museum of Natural History Fort Hays State University 3000 Sternberg Drive Hays, Kansas 67601 Front cover images of an Eastern Collared Lizard (Crotaphytus collaris) and Cajun Chorus Frog (Pseudacris fouquettei) by Suzanne L. -
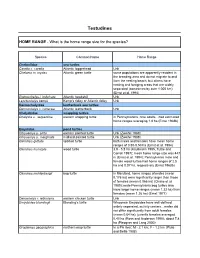
Home Range Testudines
Testudines HOME RANGE - What is the home range size for the species? Species Common Name Home Range Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle some populations are apparently resident in the breeding area and do not migrate to and from the nesting beach, but others have nesting and foraging areas that are widely separated (sometimes by over 1,000 km) (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill Unk Lepidochelys kempii Kemp’s ridley or Atlantic ridley Unk Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback Unk Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle in Pennsylvanina, nine adults…had estimated home ranges averaging 1.8 ha (Ernst 1968b) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk (Zweifel 1989) Chrysemys p. marginata midland painted turtle Unk (Zweifel 1989) Clemmys guttata spotted turtle both males and females have mean home ranges of 0.50-0.53 ha (Ernst et al. 1994) Clemmys insculpta wood turtle 3.9 - 5.8 ha (Kaufmann 1995, Tuttle and Carroll 1997); mean home range size was 447 m (Ernst et al. 1994); Pennsylvania male and female wood turtles had home ranges of 2.5 ha and 0.07 ha, respectively (Ernst 1968b) Clemmys muhlenbergii bog turtle in Maryland, home ranges of males (mean 0.176 ha) were significantly larger than those of females (mean 0.066 ha) (Chase et al. 1989); male Pennsylvania bog turtles also have larger home ranges (mean 1.33 ha) than females (mean 1.26 ha) (Ernst 1977) Deirochelys r. -

Turtles of the World, 2010 Update: Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation ProjectTurtles of the IUCN/SSC of the World Tortoise – 2010and Freshwater Checklist Turtle Specialist Group 000.85 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson, and R.A. Mittermeier, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.000.checklist.v3.2010 © 2010 by Chelonian Research Foundation • Published 14 December 2010 Turtles of the World, 2010 Update: Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status TUR T LE TAXONOMY WORKING GROUP * *Authorship of this article is by this working group of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, which for the purposes of this document consisted of the following contributors: ANDERS G.J. RHODIN 1, PE T ER PAUL VAN DI J K 2, JOHN B. IVERSON 3, AND H. BRADLEY SHAFFER 4 1Chair, IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Chelonian Research Foundation, 168 Goodrich St., Lunenburg, Massachusetts 01462 USA [[email protected]]; 2Deputy Chair, IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, Virginia 22202 USA [[email protected]]; 3Department of Biology, Earlham College, Richmond, Indiana 47374 USA [[email protected]]; 4Department of Evolution and Ecology, University of California, Davis, California 95616 USA [[email protected]] AB S T RAC T . – This is our fourth annual compilation of an annotated checklist of all recognized and named taxa of the world’s modern chelonian fauna, documenting recent changes and controversies in nomenclature, and including all primary synonyms, updated from our previous three checklists (Turtle Taxonomy Working Group [2007b, 2009], Rhodin et al. -

Curriculum Vitae
CURRICULUM VITAE Michael Joseph Dreslik Illinois Natural History Survey, 1816 South Oak Street, Champaign, IL 61820 (217)259-9135, email – [email protected] PERSONAL Born 22 August 1972 Flushing, Queens, New York AREAS OF RESEARCH INTEREST Conservation, Population, Spatial, Behavioral, Community, and Physiological Ecology of Reptiles and Amphibians EDUCATION DEGREES EARNED PH.D. University of Illinois Urbana–Champaign, Major – Natural Resource Ecology and Conservation, 1/97 – 12/05, Major Professor – Dr. C. A. Phillips Dissertation: Ecology of the Eastern Massasauga rattlesnake (Sistrurus c. catenatus) from Carlyle Lake, Clinton County, Illinois. University of Illinois Urbana–Champaign, Urbana, Illinois. xxxvii+353 pp. M.SC. Eastern Illinois University, Major – Biology, 8/94 – 5/96, GPA 3.7(4.0), Major Professor – Dr. E. O. Moll Thesis: Ecology and community relationships of the River Cooter, Pseudemys concinna, in a southern Illinois backwater. Eastern Illinois University, Charleston, Illinois. v + 69pp. B.Sc. Eastern Illinois University, Major – Zoology, 8/92 – 5/94, A.Sc. Lake Land Community College, Major – Biology, Education, 8/90 – 5/92 Cum Laude WORK EXPERIENCE POSITIONS HELD 1/2015 – Present Urban Biotic Assessment Program Coordinator – Illinois Natural History Survey, Prairie Research Institute, University of Illinois Urbana–Champaign, Illinois. 7/2008 – Present Academic Professional – Herpetologist – Illinois Natural History Survey, Prairie Research Institute, University of Illinois Urbana–Champaign, Illinois. 1/2007 – 6/2008 Assistant Research Scientist – Illinois Natural History Survey, Division of Biodiversity and Ecological Entomology, Champaign, Illinois. 7/2005 – 12/2006 Assistant Technical Scientist II – Illinois Natural History Survey, Center for Biodiversity, Champaign, Illinois. 1/2004 – 7/2005 Field Ecologist – Illinois Natural History Survey, Center for Biodiversity, Champaign, Illinois. -

Turtles of Waller Mill Lake
Turtles of Waller Mill Lake Kimberly Demnicki Zahn Department of Biology Thomas Nelson Community College 99 Thomas Nelson Drive Hampton, Virginia 23666 W. Wyatt Hoback Department of Biology Bruner Hall of Science University of Nebraska at Kearney Kearney, Nebraska 68849 Introduction Artificial freshwater lakes are constructed for a variety of purposes. They serve as storm water retention basins, drinking water reservoirs, and are used for a wide range of recreational activities (Cole 1994). In addition, these lakes provide important habitat to many vertebrate species, particularly fish, aquatic reptiles and amphibians. Of the approximately 450 herpetofaunal species found in the United States, half occur in the Southeast with about twenty percent endemic to the region (Conant and Collins 1998; Tuberville et al. 2005). Due to the abundance and diversity of herpetofauna in both terrestrial and freshwater ecosystems, they are considered an excellent ecological indicator of the health of a habitat (Tuberville et al. 2005). In fact, the biomass of the herpetofauna is typically significantly greater than that of endotherms (Iverson 1982; Tuberville et al. 2005). Often, turtles compose a major portion of the vertebrate biomass in freshwater habitats (Congdon et al. 1986). Since standing crop biomass of turtles can be so high, it is likely that they play a significant role in energy flow and nutrient cycling (Bury 1979). The success of turtles is due, in part, to the fact that some species are extremely tolerant of human-impacted ecosystems and thrive in highly modified habitats, even when other organisms are significantly negatively impacted (Mitchell 1988; Conner et al. 2005). Several species of emydid turtles are known to inhabit lakes in southeastern Virginia. -

March 2015 Page 2
I SSUE F ORTY S EVEN BIRDING SKILLS WORKSHOP FOR VOLUNTEERS—A FUN & EDUCATIONAL DAY M ARCH 2 0 1 5 Story by Kathy Woodward, Friends Board Member & Volunteer; Photos by Jane Bell, Volunteer H i g h l i g h t s ery soon, many of our volun- Great Swamp Strike Team 4 teers at Great Swamp NWR will It’s More Than Just a Duck have a new Birds bar on their 6 V Stamp volunteer vest. The bars hanging from Red Knot Gains Protection 7 their Swamp Nature Guide pin are earned by participating in training on a Meet—Jim Mulvey 9 specific subject. Red-eared Sliders 10 On Saturday, January 31, 2015, 32 vol- unteers learned about birds and birding Board of Directors so they can interact with Refuge visitors with new knowledge and skills. Mike Elaine Seckler President Anderson, Director at NJ Audubon’s Scherman Hoffman Wildlife Sanctuary, Susan Garretson Friedman Volunteer George Helmke covers the history of optics Vice-President taught how to identify many New Jersey Kathy Woodward birds. In the afternoon, Friends volunteer veterans to watch birds at the VA Hospital in Secretary George Helmke presented information about Lyons. Others will lead bird walks and build Laurel Gould the history and use of optics. Mini-sessions, awareness of the habitats at the Refuge. Treasurer led by volunteers, covered the practice of John Berry observation skills, eBird Trail Tracker, and a Mary Beth Hansbury said she really enjoyed discussion of field guides and bird apps. learning interesting tidbits to share with visi- Rich Dufort tors. -

Reptile and Amphibian Abundance and Distribution Survey Oak Ridge National Environmental Research Park 2007–2009
DOCUMENT AVAILABILITY Reports produced after January 1, 1996, are generally available free via the U.S. Department of Energy (DOE) Information Bridge. Web site http://www.osti.gov/bridge Reports produced before January 1, 1996, may be purchased by members of the public from the following source. National Technical Information Service 5285 Port Royal Road Springfield, VA 22161 Telephone 703-605-6000 (1-800-553-6847) TDD 703-487-4639 Fax 703-605-6900 E-mail [email protected] Web site http://www.ntis.gov/support/ordernowabout.htm Reports are available to DOE employees, DOE contractors, Energy Technology Data Exchange (ETDE) representatives, and International Nuclear Information System (INIS) representatives from the following source. Office of Scientific and Technical Information P.O. Box 62 Oak Ridge, TN 37831 Telephone 865-576-8401 Fax 865-576-5728 E-mail [email protected] Web site http://www.osti.gov/contact.html This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. -
Diane C. Tulipani, Ph.D. CBNERRS Discovery Lab July 15, 2014
TURTLES Diane C. Tulipani, Ph.D. CBNERRS Discovery Lab July 15, 2014 How Would You Describe a Turtle? • Reptile • Special bony or cartilaginous shell formed from ribs • Scaly skin • Exothermic (“cold-blooded”) • Breathe air • Aquatic & terrestrial • Lay eggs on land • Herbivores, carnivores, & omnivores Are They Turtles, Tortoises, & Terrapins? • All refer to species within the supraorder Chelonia • Turtle refers to all species in general • Tortoise • usually refers to any land-dwelling, non-swimming chelonian • Terrapin based on Algonquian for “edible turtle” From Around the World Pig-nosed Turtle Big-headed Turtle Spiny Soft-shelled Turtle Spiny Turtle Ancient group of reptiles Fossil turtle (~220 million years old) Modern turtle skeleton Odontochelys semitestacea Found in China Important Modification for Shell Development Two Groups based on Neck Retraction: Cryptodira draw their necks in while contracting it under their spine Pleurodira contract their necks to the side Turtles of Virginia (www.virginiaherpetologicalsociety.org ) 1. Trachemys scripta troostii (Cumberland Slider) only in southwestern VA 2. Trachemys scripta scripta (Yellow-bellied Slider) southeaster VA & near DC 3. Trachemys scripta elegans (Red-eared Slider) spotty distribution central-eastern VA 4. Terrapene carolina carolina (Eastern Box Turtle) all throughout VA 5. Sternotherus odoratus (Eastern Musk Turtle) all throughout VA 6. Sternotherus minor peltifer (Stripe-necked Musk Turtle) extreme southwestern point of VA 7. Pseudemys rubriventris (Northern Red-bellied Cooter) all of eastern VA 8. Pseudemys concinna floridana (Coastal Plain Cooter) extreme southeastern VA 9. Pseudemys concinna concinna (Eastern River Cooter) central & southwestern VA 10. Malaclemys terrapin terrapin (Northern diamondback terrapin) coastal eastern VA 11. Kinosternon subrubrum subrubrum (Eastern Mud Turtle) central to eastern VA 12. -
Trachemys Scripta Elegans (Red-Eared Slider) Management Information
Trachemys scripta elegans (Red-eared Slider) Management Information Contents 1.0 Introduction…………………………………………………………………… Page 1 2.0 Preventative Measures……………………………………………………….. Page 1 3.0 Physical Control………………………………………………………………. Page 2 4.0 Education and Awareness……………………………………………………. Page 2 5.0 Knowledge and Research…………………………………………………….. Page 3 6.0 Integrated Management……………………………………………………… Page 4 7.0 References……………………………………………………………………... Page 4 1.0 Introduction In the context of threatened and endangered species the introduction of alien turtle species should always be strongly discouraged and governments should prevent the importation of freshwater turtles (Cadi and Joly 2003). Aquatic pest animals are difficult to detect; red-eared sliders require labour-intensive methods of detection. Control of this pest requires research into novel methods of detection, such as the use of sniffer dogs (DNRW 2006c, in Wilson Wilke & Saraç 2009). Based on the results of several studies that demonstrated the deleterious impact of exotic species on local biodiversity throughout the world (eg: Jones et al. 2008), urban managers are encouraged to remove these species as soon as they are detected. However, the decision to remove exotic species may face opposition from the public which haves social and cultural perceptions of animals, plants and nature that may be different from scientific views (Minteer & Collins, 2005b, in Teillac-Deschamps et al. 2009). For a discussion of these issues please see Teillac-Deschamps and colleagues (2009); the authors have created a process-based model for the management of feral exotic pets in urban green spaces that incorporates ecological considerations, human behaviours and stakeholders and their relationships. 2.0 Preventative Measures With effect from 22 December 1997 the EU banned the import of the subspecies T.