Synthesis of Polyrotaxanes Containing Cucurbituril
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cucurbituril Assembly and Uses Thereof Cucurbiturilanordnung Und Seine Verwendungen Ensemble De Cucurbituril Et Ses Utilisations
(19) TZZ_Z__T (11) EP 1 668 015 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: C07D 487/22 (2006.01) C07D 519/00 (2006.01) of the grant of the patent: G01N 33/00 (2006.01) C08G 73/00 (2006.01) 21.08.2013 Bulletin 2013/34 (86) International application number: (21) Application number: 04770467.1 PCT/IL2004/000796 (22) Date of filing: 05.09.2004 (87) International publication number: WO 2005/023816 (17.03.2005 Gazette 2005/11) (54) CUCURBITURIL ASSEMBLY AND USES THEREOF CUCURBITURILANORDNUNG UND SEINE VERWENDUNGEN ENSEMBLE DE CUCURBITURIL ET SES UTILISATIONS (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR WO-A-00/68232 WO-A-03/004500 HU IE IT LI LU MC NL PL PT RO SE SI SK TR WO-A-03/055888 WO-A-03/055888 (30) Priority: 04.09.2003 US 499735 P • JON, SANG YONG ET AL: "Facile Synthesis of 13.01.2004 US 535829 P Cucurbit[n]uril Derivatives via Direct Functionalization: Expanding Utilization of (43) Date of publication of application: Cucurbit[n]uril" JOURNAL OF THE AMERICAN 14.06.2006 Bulletin 2006/24 CHEMICAL SOCIETY , 125(34), 10186-10187 CODEN: JACSAT; ISSN: 0002-7863, 8 June 2003 (73) Proprietor: TECHNION RESEARCH AND (2003-06-08), XP002316247 DEVELOPMENT FOUNDATION, LTD. • JON, SANG YONG ET AL: "Facile Synthesis of Haifa 32000 (IL) Cucurbit[n]uril Derivatives via Direct Functionalization: Expanding Utilization of (72) Inventor: KEINAN, Ehud Cucurbit[n]uril" JOURNAL OF THE AMERICAN 23 840 Timrat (IL) CHEMICAL SOCIETY , 125(34), 10186-10187 CODEN: JACSAT; ISSN: 0002-7863, 2003, (74) Representative: Dennemeyer & Associates S.A. -

Study on the Complexation of Macromolecule Cucurbituril with Metals and Acetamide
International Journal of Chemistry and Applications. ISSN 0974-3111 Volume 4, Number 3 (2012), pp. 219-226 © International Research Publication House http://www.irphouse.com Study on the Complexation of Macromolecule Cucurbituril with Metals and Acetamide T. Jaba Priya, Jasmine Rebecca and R. Wilfred Sugumar Department of Chemistry, Madras Christian College (Autonomous), Tambaram, Chennai-600059, India. E-mail: [email protected]; [email protected] Abstract Cucurbituril is a remarkably robust macro cyclic host molecule that has a rigid cavity capable of binding small guests under a variety of conditions. In the present study, macromolecule cucurbit[6]uril (CB[6]) and its complexes with metal ions lithium, cerium were synthesized. Mixed ligand complexes containing CB[6] and acetamide with central metal ions such as lithium were also prepared. The UV – visible spectrum of the complex of lithium with CB[6] shows a peak at 225nm. The mixed ligand complex of lithium with CB[6] and acetamide shows a sharp peak at 220nm which indicates the presence of amide functional group. The peak at 274nm indicates the presence of – NO2 group. UV-visible spectrum of the complex of cerium with CB[6] shows a peak at 213.17nm, which indicates the presence of – CONH2 group. The IR spectrum of Cucurbituril shows the following peaks 3436.96 cm-1 , 2931.16 to 2372.15 cm-1 and a sharp peak at 1726.39 cm-1 these frequencies indicate the presence of CO - N ,C-H and C=O stretching respectively. The IR spectra of the complex of Lithium with CB[6] and Cerium with CB[6] show significant variations especially around 3000 cm-1 and between 1700 to 1200 cm-1 indicating prevalence of enhanced hydrogen bonding. -
![Cucurbit[7]Uril Host-Viologen Guest Complexes](https://docslib.b-cdn.net/cover/3644/cucurbit-7-uril-host-viologen-guest-complexes-423644.webp)
Cucurbit[7]Uril Host-Viologen Guest Complexes
CUCURBIT[7]URIL HOST-VIOLOGEN GUEST COMPLEXES: ELECTROCHROMIC AND PHOTOCHEMICAL PROPERTIES by MARINA FREITAG A dissertation submitted to the Graduate School – Newark Rutgers, The State University of New Jersey in partial fulfillment of requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry Written under the direction of Professor Elena Galoppini and approved by ________________________ ________________________ ________________________ ________________________ Newark, New Jersey October, 2011 ABSTRACT OF THE DISSERTATION Abstract Cucurbituril[7] Host - Viologen Guest Complexes: Electrochromic and Photochemical Properties By MARINA FREITAG Dissertation Director: Professor Elena Galoppini In this thesis, we demonstrated that a molecular host, cucurbit[7]uril, provides an alternative method of adsorbing molecules on semiconductors and shields the guest from the hetereogenous interface. These novel hybrid systems exhibited photophysical and electrochemical properties that differ from the properties of layers obtained by directly attaching the chromophore to the semiconductor through binding groups. This thesis describes the host-guest chemistry between cucurbit[7]uril (CB[7]) and various series of viologen guests. Methylviologen (1,1'-dimethyl-4,4'-bipyridinium dichloride, MV2+), 1-methyl-1'-p-tolyl-4,4'-bipyridinium dichloride (MTV2+), and 1,1'-di- p-tolyl-(4,4'-bipyridine)-1,1'-diium dichloride (DTV2+) were encapsulated in the macrocyclic host cucurbit[7]uril, CB[7]. The complexes MV2+@CB[7] and MTV2+@CB[7] were physisorbed to the surface of 1 TiO2 nanoparticle films. The complexation into CB[7] was monitored by H NMR. TiO2 films functionalized with the complexes were studied by FT-IR-ATR and UV-Vis ii absorption. The electrochemical and spectroelectrochemical properties of MV2+@CB[7] and MTV2+@CB[7] were studied in solution and in electrochromic windows (ECDs), where the complexes were bound to TiO2 films cast on FTO. -

Formation of Functionalized Supramolecular Metallo-Organic Oligomers with Cucurbituril a Thesis Presented to the Faculty Of
Formation of Functionalized Supramolecular Metallo-organic Oligomers with Cucurbituril A thesis presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Master of Science Ian M. Del Valle December 2015 © 2015 Ian M. Del Valle. All Rights Reserved. 2 This thesis titled Formation of Functionalized Supramolecular Metallo-organic Oligomers with Cucurbituril by IAN M. DEL VALLE has been approved for the Department of Chemistry and Biochemistry and the College of Arts and Sciences by Eric Masson Associate Professor of Chemistry and Biochemistry Robert Frank Dean, College of Arts and Sciences 3 Abstract DEL VALLE, IAN M., M.S., December 2015, Chemistry Formation of Functionalized Supramolecular Metallo-organic Oligomers with Cucurbituril Director of Thesis: Eric Masson The goal of this project is to functionalize supramolecular oligomer chains with amino acids and nucleic acids in order to observe interactions with proteins and DNA. Chiral substituents are also desirable to induce helicality in the oligomer much like DNA. We explored different pathways to afford these oligomers. The first project involves forming metallo-organic oligomers using non-covalent bonds and then functionalizing them. We synthesize ligands and use alkyne-azide cycloadditions to functionalize them. These ligands can then be coordinated to various transition metals. The aromatic regions of these oligomers can then self-assemble into tube-like chains with the participation of cucurbit[8]uril. Second, we explore an alternate pathway to form functionalized chains. This second set of chains coupled amines with carboxylic acid groups attached to the ligands. This project hopes to avoid solubility problems experienced with the first project. -

Weak Functional Group Interactions Revealed Through Metal-Free Active Template Rotaxane Synthesis
ARTICLE https://doi.org/10.1038/s41467-020-14576-7 OPEN Weak functional group interactions revealed through metal-free active template rotaxane synthesis Chong Tian 1,2, Stephen D.P. Fielden 1,2, George F.S. Whitehead 1, Iñigo J. Vitorica-Yrezabal1 & David A. Leigh 1* 1234567890():,; Modest functional group interactions can play important roles in molecular recognition, catalysis and self-assembly. However, weakly associated binding motifs are often difficult to characterize. Here, we report on the metal-free active template synthesis of [2]rotaxanes in one step, up to 95% yield and >100:1 rotaxane:axle selectivity, from primary amines, crown ethers and a range of C=O, C=S, S(=O)2 and P=O electrophiles. In addition to being a simple and effective route to a broad range of rotaxanes, the strategy enables 1:1 interactions of crown ethers with various functional groups to be characterized in solution and the solid state, several of which are too weak — or are disfavored compared to other binding modes — to be observed in typical host–guest complexes. The approach may be broadly applicable to the kinetic stabilization and characterization of other weak functional group interactions. 1 Department of Chemistry, University of Manchester, Manchester M13 9PL, UK. 2These authors contributed equally: Chong Tian, Stephen D. P. Fielden. *email: [email protected] NATURE COMMUNICATIONS | (2020) 11:744 | https://doi.org/10.1038/s41467-020-14576-7 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-14576-7 he bulky axle end-groups of rotaxanes mechanically lock To explore the scope of this unexpected method of rotaxane Trings onto threads, preventing the dissociation of the synthesis, here we carry out a study of the reaction with a series of components even if the interactions between them are not related electrophiles. -

Synthesis and Characterization of Supramolecular Cucurbituril, Molybdenum 2,2'-Bipyridyl Complex
International Journal of Scientific & Engineering Research Volume 8, Issue 10, October-2017 140 ISSN 2229-5518 SYNTHESIS AND CHARACTERIZATION OF SUPRAMOLECULAR CUCURBITURIL, MOLYBDENUM 2,2’-BIPYRIDYL COMPLEX Ms. Anitha. V1 , Dr. Nandagopal. J2, Ms. Ramanachiar.R3, Ms. Sasikala.S4, Ms. Ariyamala .R5 Department of chemistry, Velammal Engineering college, Chennai, Tamilnadu. Abstract Supra molecular Cucurbituril, Molybdenum2,2’-bipyridyl complex has been synthesized by precipitation followed by slow evaporation techniques. Changes in the physical properties of the ligand and the complex were characterized by melting point and solubility test . The absorption spectrum of the Cucurbituril, Molybdenum2,2’-bipyridyl complex were studied by decreasing and increasing concentrations by using UV – Visible spectrophotometer and IR spectrophotometer. The interaction between the metal and ligands were studied by UV- visible spectral analysis. The functional groups and modes of vibration were identified by IR spectral analysis. Key words: Cucurbituril, evaporation, precipitation, absorption, ligand, spectrophotometer. INTRODUCTION complex of Cucurbit[6]uril which incorporates the p-Xylene diammonium cation into the macrocyclic A Supramolecular assembly or “supramolecule” is a cavity. Researchers have shown that Cucurbit[6]uril well defined complex of molecules held together by acts as a catalyst in cyclo-addition reactions. noncovalent bonds. Cucurbiturils are macrocyclic Detailed examination has been done on the molecules consisting of glycouril repeating units. construction of supramolecular organic, inorganic These supramolecular compounds are particularly compounds from macrocyclic cavitand interesting to chemists because they are molecular cucurbit[6]Uril and mono and polynuclear aqua containers that are capable of binding other complexes. Cucurbit[6]uril is used in the molecules within their cavities. -

Host-Guest Compounds Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications Xue Yang, Ruibing Wang, Anthony Kermagoret, David Bardelang
Host-Guest Compounds Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications Xue Yang, Ruibing Wang, Anthony Kermagoret, David Bardelang To cite this version: Xue Yang, Ruibing Wang, Anthony Kermagoret, David Bardelang. Host-Guest Compounds Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications. Ange- wandte Chemie International Edition, Wiley-VCH Verlag, 2020, pp.2-15. 10.1002/anie.202004622. hal-02972332 HAL Id: hal-02972332 https://hal-amu.archives-ouvertes.fr/hal-02972332 Submitted on 23 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications. Xue Yang,[a] Ruibing Wang,*[b] Anthony Kermagoret,*[a] and David Bardelang*[a] Abstract: Proteins are an endless source of inspiration. By carefully Xue Yang earned her Master’s degree from tuning the amino-acid sequence of proteins, nature made them evolve the University of Macau (China) in 2017. She from primary to quaternary structures, a property specific to protein is currently pursuing her PhD on the oligomers and often crucial to accomplish their function. On the other supramolecular chemistry of cucurbiturils at Aix- hand, the synthetic macrocycles cucurbiturils (CBs) have shown Marseille University. -
![Host-Guest Chemistry Between Cucurbit[7]Uril and Neutral and Cationic Guests](https://docslib.b-cdn.net/cover/2357/host-guest-chemistry-between-cucurbit-7-uril-and-neutral-and-cationic-guests-1532357.webp)
Host-Guest Chemistry Between Cucurbit[7]Uril and Neutral and Cationic Guests
HOST-GUEST CHEMISTRY BETWEEN CUCURBIT[7]URIL AND NEUTRAL AND CATIONIC GUESTS by Ian William Wyman A thesis submitted to the Department of Chemistry In conformity with the requirements for the degree of Doctor of Philosophy. Queen’s University Kingston, Ontario, Canada (January, 2010) Copyright © Ian William Wyman, 2010 Dedicated in memory of my Grandfather, Norman Brannen, 1917-2009. ii Abstract This thesis describes the host-guest chemistry between cucurbit[7]uril (CB[7]) and various series of guests, including neutral polar organic solvents, bis(pyridinium)alkane dications, local anaesthetics, acetylcholine analogues, as well as succinylcholine and decamethonium analogues, in aqueous solution. A focus of this thesis is the effects of varying the chemical structures within different series of guests upon the nature of the host-guest chemistry, such as the relative position and orientation of the guest relative to the CB[7] cavity, and the strengths of the binding affinities. The binding affinities of polar organic solvents with CB[7] depend upon the hydrophobic effect and dipole-quadrupole interactions. The polar guests align themselves so that their dipole moment is perpendicular to the quadrupole moment of CB[7]. The binding strengths of acetone and acetophenone to CB[7] decrease in the presence of alkali metals. Discrete 1:1 and 2:1 host-guest complexes are formed between CB[7] and a series of α,ω -bis(pyridinium)alkane guests. In most cases the CB[7] initially occupies the aliphatic linker when the 1:1 complex is formed and migrates to the terminal regions as the second CB[7] is added. -

Cucurbiturils As Molecular Containers: the Mechanism Of
Cucurbiturils as Molecular Containers: The Mechanism of Complexation of Small Guests, the Effects of the Inclusion on their Photophysical Properties, and Potential Applications A thesis presented by César Augusto Márquez González to the School of Engineering and Science in fulfillment of the requirements for the degree of Doctor of Chemistry in the graduate program of Nanomolecular Sciences International University Bremen Bremen, 2003 Approved by the School of Engineering and Science by request of Prof. Dr. Hans-Jürgen Buschmann, Prof. Dr. Ulrich Kortz, Prof. Dr. Werner M. Nau, Prof. Dr. Thomas Nugent, and Prof. Dr. Ryan M. Richards Bremen, 17 December, 2003 Prof. Dr. Gerhard Haerendel Dean 2 Contents 1. Introduction 11 1.1. Supramolecular Chemistry 11 1.2. Cucurbiturils as Molecular Containers 16 1.3. Scope of Dissertation 22 2. Complexation Mechanism 26 2.1. Two Mechanisms of Slow Host-Guest Complexation between Cucurbit[6]uril and Cyclohexylmethylamine: pH-Responsive Supramolecular Kinetics 26 2.2. The Mechanism of Host-Guest Complexation by Cucurbit[6]uril 29 3. Fluorescent Host-Guest Systems 31 3.1. 2,3-Diazabicyclo[2.2.2]oct-2-ene in Supramolecular Chemistry 31 3.2. Polarizabilities Inside Molecular Containers 34 4. Potential Applications 37 4.1. Selective Fluorescence Quenching of 2,3-Diazabicyclo[2.2.2]oct-2-ene by Nucleotides 37 4.2. Cucurbiturils: Molecular Nanocapsules for Time-Resolved Fluorescence- based Assays 40 3 5. Ongoing Projects 44 5.1. The Cucurbit[7]uril•2,3-Diazabicyclo[2.2.2]oct-2-ene Host-Guest System. Influence of Associated Metal Ions on its Photophysical Properties 45 5.2. -
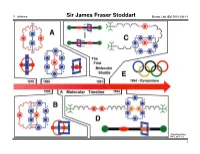
Sir James Fraser Stoddart Baran Lab GM 2010-08-14
Y. Ishihara Sir James Fraser Stoddart Baran Lab GM 2010-08-14 (The UCLA USJ, 2007, 20, 1–7.) 1 Y. Ishihara Sir James Fraser Stoddart Baran Lab GM 2010-08-14 (The UCLA USJ, 2007, 20, 1–7.) 2 Y. Ishihara Sir James Fraser Stoddart Baran Lab GM 2010-08-14 Professor Stoddart's publication list (also see his website for a 46-page publication list): - 9 textbooks and monographs - 13 patents "Chemistry is for people - 894 communications, papers and reviews (excluding book chapters, conference who like playing with Lego abstracts and work done before his independent career, the tally is about 770) and solving 3D puzzles […] - At age 68, he is still very active – 22 papers published in the year 2010, 8 months in! Work is just like playing - He has many publications in so many fields... with toys." - Journals with 10+ papers: JACS 75 Acta Crystallogr Sect C 26 ACIEE 67 JCSPT1 23 "There is a lot of room for ChemEurJ 62 EurJOC 19 creativity to be expressed JCSCC 51 ChemComm 15 in chemis try by someone TetLett 42 Carbohydr Res 12 who is bent on wanting to OrgLett 35 Pure and Appl Chem 11 be inventive and make JOC 28 discoveries." - High-profile general science journals: Nature 4 Science 5 PNAS 8 - Reviews: AccChemRes 8 ChemRev 4 ChemSocRev 6 - Uncommon venues of publication for British or American scientists: Coll. Czechoslovak Chem. Comm. 5 Mendeleev Communications 2 Israel Journal of Chemistry 5 Recueil des Trav. Chim. des Pays-Bas 2 Canadian Journal of Chemistry 4 Actualité chimique 1 Bibliography (also see his website, http://stoddart.northwestern.edu/ , for a 56-page CV): Chemistry – An Asian Journal 3 Bulletin of the Chem. -

Rotaxanes and Catenanes by Click Chemistry
Mini Review Rotaxanes and Catenanes by Click Chemistry Ognjen Sˇ. Miljanic´a, William R. Dichtela, b, Ivan Aprahamiana, Rosemary D. Rohdeb, Heather D. Agnewb, James R. Heathb* and J. Fraser Stoddarta* a California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, California 90095, USA, E-mail: [email protected] b Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA, E-mail: [email protected] Keywords: Catenanes, Click chemistry, Interlocked molecules, Rotaxanes, Self-assembly, Surface chemistry Received: June 1, 2007; Accepted: July 11, 2007 DOI: 10.1002/qsar.200740070 Abstract Copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition between terminal alkynes and azides – also known as the copper (Cu)-catalyzed Azide-Alkyne Cycloaddition (CuAAC) – has been used in the syntheses of molecular compounds with diverse structures and functions, owing to its functional group tolerance, facile execution, and mild reaction conditions under which it can be promoted. Recently, rotaxanes of four different structural types, as well as donor/acceptor catenanes, have been prepared using CuAAC, attesting to its tolerance to supramolecular interactions as well. In one instance of a rotaxane synthesis, the catalytic role of copper has been combined successfully with its previously documented ability to preorganize rotaxane precursors, i.e., form pseudoro- taxanes. The crystal structure of a donor/acceptor catenane formed using the CuAAC reaction indicates that any secondary [p···p] interactions between the 1,2,3-triazole ring and the bipyridinium p-acceptor are certainly not destabilizing. Finally, the preparation of robust rotaxane and catenane molecular monolayers onto metal and semiconductor surfaces is premeditated based upon recent advances in the use of the Huisgen reaction for surface functionalization. -

How Molecules Became Machines
THE NOBEL PRIZE IN CHEMISTRY 2016 POPULAR SCIENCE BACKGROUND How molecules became machines The Nobel Prize in Chemistry 2016 is awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart and Bernard L. Feringa for their development of molecular machines that are a thousand times thinner than a hair strand. This is the story of how they succeeded in linking molecules together to design everything from a tiny lift to motors and miniscule muscles. How small can you make machinery? This is the question that Nobel Laureate Richard Feynman, famed for his 1950s’ predictions of developments in nanotechnology, posed at the start of a visionary lecture in 1984. Barefoot, and wearing a pink polo top and beige shorts, he turned to the audience and said: “Now let us talk about the possibility of making machines with movable parts, which are very tiny.” He was convinced it was possible to build machines with dimensions on the nanometre scale. These already existed in nature. He gave bacterial flagella as an example, corkscrew-shaped macromole- cules which, when they spin, make bacteria move forward. But could humans – with their gigantic hands – build machines so small that you would need an electron microscope to see them? A vision of the future – molecular machines will exist within 25–30 years One possible way would be to build a pair of mechanical hands that are smaller than your own, which in turn build a pair of smaller hands, which build even smaller hands, and so on, until a pair of miniscule hands can build equally miniscule machinery.