Life in a Drop of Water (6-12)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skyline Park Teacher's Guide Glossary
Skyline Park Teacher’s Guide Cfwep.Org • Clark Fork Watershed Education Program Skyline Park • Butte, Montana Skyline Park Teacher’s Guide Table of Contents Chapter I. Introduction and Welcome 1. Overview of Skyline Park 2. Map of Skyline Park 3. How to use this Guide and the Activities II. About the NGSS and Guide Alignment 1. Background of NGSS and the K-12 Framework 2. Skyline Park Guide and the Montana Content Standards for Science Instruction III. Building Your Content Knowledge 1. Site History 2. Habitat Types of Skyline Park 3. Pond Life 4. Water Quality 5. Stormwater 6. Seed Pod Islands and Pollinators 7. Geology and Soils 8. Restoration IV. Activities 1. Science Note Booking 1.1: Science Note Booking About Skyline Park 2. History 2.1: Write Your Own History for this Site 2.2: Create a Timeline for this Area 3. Mapping the Skyline Park Site 3.1: Where is That?: Mapping the Skyline Park Site 4. Pond Life – Microbes 4.1: Wanderers, Drifters and Floaters, Oh My! 5. Pond Life – Aquatic Macroinvertebrates 2 Skyline Park Teacher’s Guide 5.1-Part 1: What’s That Squirming and Swimming in the Water? Collection and Identification of Pond Macroinvertebrates 5.1-Part 2: What are Those Macroinvertebrates Telling Us? Aquatic Macros as Biological Indicators 5.2: Who's My Mommy? Matching Aquatic Insect Young with their Adult Form 6. Pond Life – Terrestrial Animals 6.1: Crunch Game! The Aquatic Food Chain Game 7. Water Quality 7.1, Option 1: What Life Can This Water Support? Water Quality - WWMD Kits and Data Upload 7.1, Option 2: Water Quality – Vernier Lab Quest Units and Probes 8. -

The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba Stagnalis N. Sp
diversity Article The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea) Anastasia S. Borodina 1,2, Alexander P. Mylnikov 1,†, Jan Janouškovec 3 , Patrick J. Keeling 4 and Denis V. Tikhonenkov 1,5,* 1 Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, 152742 Borok, Russia; [email protected] 2 Department of Zoology and Parasitology, Voronezh State University, Universitetskaya Ploshad 1, 394036 Voronezh, Russia 3 Centre Algatech, Laboratory of Photosynthesis, Institute of Microbiology, Czech Academy of Sciences, Opatovický Mlýn, 37981 Tˇreboˇn,Czech Republic; [email protected] 4 Department of Botany, University of British Columbia, 6270 University Boulevard, Vancouver, BC V6T1Z4, Canada; [email protected] 5 AquaBioSafe Laboratory, University of Tyumen, 625003 Tyumen, Russia * Correspondence: [email protected]; Tel.: +7-485-472-4533 † Alexander P. Mylnikov is deceased. http://zoobank.org/References/e543a49a-16c1-4b7c-afdb-0bc56b632ef0 Abstract: Heterolobose amoebae are important members of marine, freshwater, and soil microbial Citation: Borodina, A.S.; Mylnikov, communities, but their diversity remains under-explored. We studied the diversity of Vahlkampfiidae A.P.; Janouškovec, J.; Keeling, P.J.; to improve our understanding of heterolobosean relationships and their representation in aquatic Tikhonenkov, D.V. The Morphology, benthos. Using light and electron microscopy, and molecular phylogenies based on the SSU rRNA Ultrastructure and Molecular and ITS loci, we describe the fine morphology and evolutionary relationships of a new heterolobosean Phylogeny of a New Freshwater Parafumarolamoeba stagnalis n. sp. from a small pond in European Russia. Cells of P. stagnalis possess Heterolobose Amoeba a clearly distinguishable anterior hyaline pseudopodium, eruptive movement, several thin and Parafumarolamoeba stagnalis n. -

Antony Van Leeuwenhoek, the Father of Microscope
Turkish Journal of Biochemistry – Türk Biyokimya Dergisi 2016; 41(1): 58–62 Education Sector Letter to the Editor – 93585 Emine Elif Vatanoğlu-Lutz*, Ahmet Doğan Ataman Medicine in philately: Antony Van Leeuwenhoek, the father of microscope Pullardaki tıp: Antony Van Leeuwenhoek, mikroskobun kaşifi DOI 10.1515/tjb-2016-0010 only one lens to look at blood, insects and many other Received September 16, 2015; accepted December 1, 2015 objects. He was first to describe cells and bacteria, seen through his very small microscopes with, for his time, The origin of the word microscope comes from two Greek extremely good lenses (Figure 1) [3]. words, “uikpos,” small and “okottew,” view. It has been After van Leeuwenhoek’s contribution,there were big known for over 2000 years that glass bends light. In the steps in the world of microscopes. Several technical inno- 2nd century BC, Claudius Ptolemy described a stick appear- vations made microscopes better and easier to handle, ing to bend in a pool of water, and accurately recorded the which led to microscopy becoming more and more popular angles to within half a degree. He then very accurately among scientists. An important discovery was that lenses calculated the refraction constant of water. During the combining two types of glass could reduce the chromatic 1st century,around year 100, glass had been invented and effect, with its disturbing halos resulting from differences the Romans were looking through the glass and testing in refraction of light (Figure 2) [4]. it. They experimented with different shapes of clear glass In 1830, Joseph Jackson Lister reduced the problem and one of their samples was thick in the middle and thin with spherical aberration by showing that several weak on the edges [1]. -

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
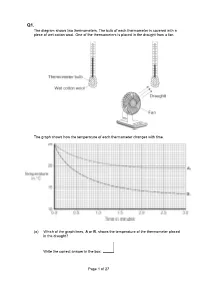
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

Burmese Amber Taxa
Burmese (Myanmar) amber taxa, on-line supplement v.2021.1 Andrew J. Ross 21/06/2021 Principal Curator of Palaeobiology Department of Natural Sciences National Museums Scotland Chambers St. Edinburgh EH1 1JF E-mail: [email protected] Dr Andrew Ross | National Museums Scotland (nms.ac.uk) This taxonomic list is a supplement to Ross (2021) and follows the same format. It includes taxa described or recorded from the beginning of January 2021 up to the end of May 2021, plus 3 species that were named in 2020 which were missed. Please note that only higher taxa that include new taxa or changed/corrected records are listed below. The list is until the end of May, however some papers published in June are listed in the ‘in press’ section at the end, but taxa from these are not yet included in the checklist. As per the previous on-line checklists, in the bibliography page numbers have been added (in blue) to those papers that were published on-line previously without page numbers. New additions or changes to the previously published list and supplements are marked in blue, corrections are marked in red. In Ross (2021) new species of spider from Wunderlich & Müller (2020) were listed as being authored by both authors because there was no indication next to the new name to indicate otherwise, however in the introduction it was indicated that the author of the new taxa was Wunderlich only. Where there have been subsequent taxonomic changes to any of these species the authorship has been corrected below. -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

A Revised Classification of Naked Lobose Amoebae (Amoebozoa
Protist, Vol. 162, 545–570, October 2011 http://www.elsevier.de/protis Published online date 28 July 2011 PROTIST NEWS A Revised Classification of Naked Lobose Amoebae (Amoebozoa: Lobosa) Introduction together constitute the amoebozoan subphy- lum Lobosa, which never have cilia or flagella, Molecular evidence and an associated reevaluation whereas Variosea (as here revised) together with of morphology have recently considerably revised Mycetozoa and Archamoebea are now grouped our views on relationships among the higher-level as the subphylum Conosa, whose constituent groups of amoebae. First of all, establishing the lineages either have cilia or flagella or have lost phylum Amoebozoa grouped all lobose amoe- them secondarily (Cavalier-Smith 1998, 2009). boid protists, whether naked or testate, aerobic Figure 1 is a schematic tree showing amoebozoan or anaerobic, with the Mycetozoa and Archamoe- relationships deduced from both morphology and bea (Cavalier-Smith 1998), and separated them DNA sequences. from both the heterolobosean amoebae (Page and The first attempt to construct a congruent molec- Blanton 1985), now belonging in the phylum Per- ular and morphological system of Amoebozoa by colozoa - Cavalier-Smith and Nikolaev (2008), and Cavalier-Smith et al. (2004) was limited by the the filose amoebae that belong in other phyla lack of molecular data for many amoeboid taxa, (notably Cercozoa: Bass et al. 2009a; Howe et al. which were therefore classified solely on morpho- 2011). logical evidence. Smirnov et al. (2005) suggested The phylum Amoebozoa consists of naked and another system for naked lobose amoebae only; testate lobose amoebae (e.g. Amoeba, Vannella, this left taxa with no molecular data incertae sedis, Hartmannella, Acanthamoeba, Arcella, Difflugia), which limited its utility. -

Experimental Listeria–Tetrahymena–Amoeba Food Chain Functioning Depends on Bacterial Virulence Traits Valentina I
Pushkareva et al. BMC Ecol (2019) 19:47 https://doi.org/10.1186/s12898-019-0265-5 BMC Ecology RESEARCH ARTICLE Open Access Experimental Listeria–Tetrahymena–Amoeba food chain functioning depends on bacterial virulence traits Valentina I. Pushkareva1, Julia I. Podlipaeva2, Andrew V. Goodkov2 and Svetlana A. Ermolaeva1,3* Abstract Background: Some pathogenic bacteria have been developing as a part of terrestrial and aquatic microbial eco- systems. Bacteria are consumed by bacteriovorous protists which are readily consumed by larger organisms. Being natural predators, protozoa are also an instrument for selection of virulence traits in bacteria. Moreover, protozoa serve as a “Trojan horse” that deliver pathogens to the human body. Here, we suggested that carnivorous amoebas feeding on smaller bacteriovorous protists might serve as “Troy” themselves when pathogens are delivered to them with their preys. A dual role might be suggested for protozoa in the development of traits required for bacterial passage along the food chain. Results: A model food chain was developed. Pathogenic bacteria L. monocytogenes or related saprophytic bacteria L. innocua constituted the base of the food chain, bacteriovorous ciliate Tetrahymena pyriformis was an intermedi- ate consumer, and carnivorous amoeba Amoeba proteus was a consumer of the highest order. The population of A. proteus demonstrated variations in behaviour depending on whether saprophytic or virulent Listeria was used to feed the intermediate consumer, T. pyriformis. Feeding of A. proteus with T. pyriformis that grazed on saprophytic bacteria caused prevalence of pseudopodia-possessing hungry amoebas. Statistically signifcant prevalence of amoebas with spherical morphology typical for fed amoebas was observed when pathogenic L. -

Can Protozoa Prove the Beginning of the World?
Southeastern University FireScholars Classical Conversations Spring 2020 Can Protozoa Prove the Beginning of the World? Karina L. Burton Southeastern University - Lakeland, [email protected] Follow this and additional works at: https://firescholars.seu.edu/ccplus Part of the Cell Biology Commons, and the Evolution Commons Recommended Citation Burton, Karina L., "Can Protozoa Prove the Beginning of the World?" (2020). Classical Conversations. 9. https://firescholars.seu.edu/ccplus/9 This Term Paper is brought to you for free and open access by FireScholars. It has been accepted for inclusion in Classical Conversations by an authorized administrator of FireScholars. For more information, please contact [email protected]. 1 Can Protozoa Prove the Beginning of the World? Karina L. Burton Classical Conversations: Challenge 4; Southeastern University ENGL 1233: English Composition II Grace Veach April 16, 2020 2 Abstract Protozoa are magnificent creatures. They exhibit all of the functions intrinsic to living organisms: irritability, metabolism, growth and reproduction. Within these functions, there are numerous examples of mutations that occur in order for organisms to adapt to their given environments. Irritability is demonstrated in protozoa by their use of pseudopodia, flagella, or cilia for motility; it has been shown that such locomotors exhibit diversity while maintaining similar protein and chemical structures that appear to be a result of evolutionary processes. Metabolism in protozoa is similar to that of larger animals, but their diet is unique. They primarily feast upon bacteria, which have begun mutating to evade easy ingestion and digestion by protozoa, therefore increasing their survival rate and making it necessary for protozoa to adapt. -

CH28 PROTISTS.Pptx
9/29/14 Biosc 41 Announcements 9/29 Review: History of Life v Quick review followed by lecture quiz (history & v How long ago is Earth thought to have formed? phylogeny) v What is thought to have been the first genetic material? v Lecture: Protists v Are we tetrapods? v Lab: Protozoa (animal-like protists) v Most atmospheric oxygen comes from photosynthesis v Lab exam 1 is Wed! (does not cover today’s lab) § Since many of the first organisms were photosynthetic (i.e. cyanobacteria), a LOT of excess oxygen accumulated (O2 revolution) § Some organisms adapted to use it (aerobic respiration) Review: History of Life Review: Phylogeny v Which organelles are thought to have originated as v Homology is similarity due to shared ancestry endosymbionts? v Analogy is similarity due to convergent evolution v During what event did fossils resembling modern taxa suddenly appear en masse? v A valid clade is monophyletic, meaning it consists of the ancestor taxon and all its descendants v How many mass extinctions seem to have occurred during v A paraphyletic grouping consists of an ancestral species and Earth’s history? Describe one? some, but not all, of the descendants v When is adaptive radiation likely to occur? v A polyphyletic grouping includes distantly related species but does not include their most recent common ancestor v Maximum parsimony assumes the tree requiring the fewest evolutionary events is most likely Quiz 3 (History and Phylogeny) BIOSC 041 1. How long ago is Earth thought to have formed? 2. Why might many organisms have evolved to use aerobic respiration? PROTISTS! Reference: Chapter 28 3.