Yobe State Project
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
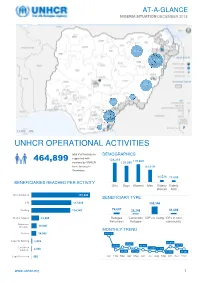
Unhcr Operational Activities 464,899
AT-A-GLANCE NIGERIA SITUATION DECEMBER 2018 28,280 388,208 20,163 1,770 4,985 18.212 177 Bénéficiaires Reached UNHCR OPERATIONAL ACTIVITIES total # of individuals DEMOGRAPHICS supported with 464,899 128,318 119,669 services by UNHCR 109,080 from January to 81,619 December; 34,825 of them from Mar-Apr 14,526 11,688 2018 BENEFICIARIES REACHED PER ACTIVITY Girls Boys Women Men Elderly Elderly Women Men Documentation 172,800 BENEFICIARY TYPE CRI 117,838 308,346 Profiling 114,747 76,607 28,248 51,698 Shelter Support 22,905 Refugee Cameroon IDPs in Camp IDPs in host Returnees Refugee community Awareness Raising 16,000 MONTHLY TREND Referral 14,956 140,116 Capacity Building 2,939 49,819 39,694 24,760 25,441 34,711 Livelihood 11,490 11,158 Support 2,048 46,139 37,118 13,770 30,683 Legal Protection 666 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec www.unhcr.org 1 NIGERIA SITUATION AT-A-GLANCE / DEC 2018 CORE UNHCR INTERVENTIONS IN NIGERIA UNHCR Nigeria strategy is based on the premise that the government of Nigeria assumes the primary responsibility to provide protection and assistance to persons of concern. By building and reinforcing self-protection mechanisms, UNHCR empowers persons of concern to claim their rights and to participate in decision-making, including with national and local authorities, and with humanitarian actors. The overall aim of UNHCR Nigeria interventions is to prioritize and address the most serious human rights violations, including the right to life and security of persons. -

North-East Nigeria January 2021
OPERATIONAL UPDATE North-East Nigeria January 2021 Over 6,100 men, women and UNHCR’s protection, human rights and UNHCR and partners raised children were newly border monitoring teams reached nearly awareness about COVID-19 and displaced in Borno, 33,000 internally displaced people and protection among over 22,000 Adamawa and Yobe States refugee returnees in Borno, Adamawa and people in the BAY States in in January. Yobe (BAY) States. January 2021. A UNHCR protection partner colleague conducts a rapid protection assessment with internally displaced people in Bama, Borno State. © UNHCR/Daniel Bisu www.unhcr.or g 1 NORTH-EAST NIGERIA OPERATIONAL UPDATE JANUARY 2021 Operational Highlights ■ The security situation in the North-East remains unpredictable. The operational area continues to be impacted by the ongoing violent conflict, terrorism, and criminal activities, which have resulted in the displacement, killing and abduction of civilians as well as the destruction of properties and critical infrastructure. The second wave of COVID-19 also continues to exacerbate the already worsening situation. A total of 43 security incidents perpetrated by NSAG in the BAY States comprised of attacks on civilians, improvised explosive devices, and attacks on security forces. ■ In Borno State, members of the non-State armed groups (NSAGs) continued their attacks on both civilian and military targets, attempted to overrun of villages and towns and mounted illegal vehicle checkpoints for the purpose of abduction, looting and robbery. The main supply routes Maiduguri- Gubio, Maiduguri-Mafa and Mungono-Ngala in the Northern axis were most severely hit. The situation along the Maiduguri-Damaturu road, a main supply route, worsened further in January, forcing the reclassification of the route from the hitherto “Restricted” to “No go” for humanitarian staff and cargo. -

Agulu Road, Adazi Ani, Anambra State. ANAMBRA 2 AB Microfinance Bank Limited National No
LICENSED MICROFINANCE BANKS (MFBs) IN NIGERIA AS AT FEBRUARY 13, 2019 S/N Name Category Address State Description 1 AACB Microfinance Bank Limited State Nnewi/ Agulu Road, Adazi Ani, Anambra State. ANAMBRA 2 AB Microfinance Bank Limited National No. 9 Oba Akran Avenue, Ikeja Lagos State. LAGOS 3 ABC Microfinance Bank Limited Unit Mission Road, Okada, Edo State EDO 4 Abestone Microfinance Bank Ltd Unit Commerce House, Beside Government House, Oke Igbein, Abeokuta, Ogun State OGUN 5 Abia State University Microfinance Bank Limited Unit Uturu, Isuikwuato LGA, Abia State ABIA 6 Abigi Microfinance Bank Limited Unit 28, Moborode Odofin Street, Ijebu Waterside, Ogun State OGUN 7 Above Only Microfinance Bank Ltd Unit Benson Idahosa University Campus, Ugbor GRA, Benin EDO Abubakar Tafawa Balewa University Microfinance Bank 8 Limited Unit Abubakar Tafawa Balewa University (ATBU), Yelwa Road, Bauchi BAUCHI 9 Abucoop Microfinance Bank Limited State Plot 251, Millenium Builder's Plaza, Hebert Macaulay Way, Central Business District, Garki, Abuja ABUJA 10 Accion Microfinance Bank Limited National 4th Floor, Elizade Plaza, 322A, Ikorodu Road, Beside LASU Mini Campus, Anthony, Lagos LAGOS 11 ACE Microfinance Bank Limited Unit 3, Daniel Aliyu Street, Kwali, Abuja ABUJA 12 Achina Microfinance Bank Limited Unit Achina Aguata LGA, Anambra State ANAMBRA 13 Active Point Microfinance Bank Limited State 18A Nkemba Street, Uyo, Akwa Ibom State AKWA IBOM 14 Ada Microfinance Bank Limited Unit Agwada Town, Kokona Local Govt. Area, Nasarawa State NASSARAWA 15 Adazi-Enu Microfinance Bank Limited Unit Nkwor Market Square, Adazi- Enu, Anaocha Local Govt, Anambra State. ANAMBRA 16 Adazi-Nnukwu Microfinance Bank Limited Unit Near Eke Market, Adazi Nnukwu, Adazi, Anambra State ANAMBRA 17 Addosser Microfinance Bank Limited State 32, Lewis Street, Lagos Island, Lagos State LAGOS 18 Adeyemi College Staff Microfinance Bank Ltd Unit Adeyemi College of Education Staff Ni 1, CMS Ltd Secretariat, Adeyemi College of Education, Ondo ONDO 19 Afekhafe Microfinance Bank Ltd Unit No. -

Growth Geographical Determinants of the Structural and Functional
Growth Vol. 1, No. 1, 10-17, 2014 http://asianonlinejournals.com/index.php/Growth Geographical Determinants of the Structural and Functional Growth of Damaturu Town in Yobe State, Nigeria Ahmed AbubakarJajere1* --- Ibrahim Jaro Musa2 --- Muhammad Isma’il3 1Department of Geography, Umar Sulaiman College of Education, Gashua 2,3Department of Geography, Ahmadu Bello University, Zaria Abstract Damaturu town became the capital of Yobe State when the state was created in 1991. Since then, the town has been experiencing rapid changes in the landuse/landcover types due to urban expansion, economic development, and social transformation in the town. This study examined the geographical determinants of the growth of Damaturu town from 1986 to 2009. The satellite imageries of Damaturu were obtained processed and analysed using Remote Sensing and Geographic Information System techniques to determine the growth rate of the town within the period of study. This was complemented with the information acquired from the field survey to achieve the objectives of the study. Findings revealed that within this period (1991-1999), Damaturu built-up area increased about four times while the urban area increased more than four times. This significant growth was influenced by the location of the administrative offices and housing estates at the periphery of the town, categorisation of the land into administrative, residential, commercial, and industrial areas; as well as the transportation network and substantial population growth within the period. The most influential change within the second period (1999-2005) was increased agriculture and significant urban expansion. Within the current period (2005-2009), the urban area expanded by about 22Km2. -

World Bank Document
PROCUREMENT PLAN (Textual Part) Project information: Country: Nigeria Public Disclosure Authorized Project Name: Multi-Sectoral Crisis Recovery Project for North East Nigeria (MCRP) P- Number: P157891 Project Implementation Agency: MCRP PCU (Federal and States) Date of the Procurement Plan: Updated -December 22, 2017. Period covered by this Procurement Plan: From 01/12/2018 – 30/06/2019. Preamble Public Disclosure Authorized In accordance with paragraph 5.9 of the “World Bank Procurement Regulations for IPF Borrowers” (July 2016) (“Procurement Regulations”) the Bank’s Systematic Tracking and Exchanges in Procurement (STEP) system will be used to prepare, clear and update Procurement Plans and conduct all procurement transactions for the Project. This textual part along with the Procurement Plan tables in STEP constitute the Procurement Plan for the Project. The following conditions apply to all procurement activities in the Procurement Plan. The other elements of the Procurement Plan as required under paragraph 4.4 of the Procurement Regulations are set forth in STEP. The Bank’s Standard Procurement Documents: shall be used for all contracts subject to international competitive procurement and those contracts as specified in the Procurement Plan tables in STEP. Public Disclosure Authorized National Procurement Arrangements: In accordance with paragraph 5.3 of the Procurement Regulations, when approaching the national market (as specified in the Procurement Plan tables in STEP), the country’s own procurement procedures may be used. When the Borrower uses its own national open competitive procurement arrangements as set forth in the FGN Public Procurement Act 2007; such arrangements shall be subject to paragraph 5.4 of the Procurement Regulations. -

Nigeria National Emergency Action Plan – January 2017
NATIONAL PRIMARY HEALTH CARE DEVELOPMENT AGENCY 2017 NIGERIA POLIO ERADICATION EMERGENCY PLAN January 2017, Abuja NPHCDA Plot 681/682 Port Harcourt Crescent Off Gimbiya street, off Ahmadu Bello Way Garki Area 11 Abuja 1 Abbreviations AFP Acute Flaccid Paralysis AVADAR Auto-Visual AFP detection and Reporting. bOPV Bivalent oral polio vaccine BMGF Bill and Melinda Gates Foundation CDC Centers for Disease Control and Prevention CJTF Civilian Joint Task Force cVDPV Circulating Vaccine Derived Poliovirus DOPV Directly observed polio vaccination EOC Emergency Operations Centre ERC Expert Review Committee of Polio Eradication and Routine Immunization EPI Expanded Programme on Immunization FCT Federal Capital Territory FMOH Federal Ministry of Health FOMWAN Federation of Muslim Women Associations in Nigeria FRR Financial Resources Requirements GAVI Global Alliance of Vaccines and Immunization ICC Inter-agency Coordination Committee IDPs Internally displaced populations IPC Inter-personal Communication IPDs Immunization Plus Days IMB Independent Monitoring Board LGA Local Government Area LQAS Lot quality assurance sampling mOPV Monovalent oral polio vaccine NCC National Certification Committee NICS National Immunization Coverage Survey NIFAA Nigeria Interfaith Action Association NPEEP National Polio Eradication Emergency Plan NTL Northern Traditional Leaders Committee on Primary Health Care Delivery NPHCDA National Primary Health Care Development Agency OPV Oral polio vaccine PEI Polio Eradication Initiative PTFoPE Presidential Task Force on Polio Eradication RES Reaching Every Settlement RI Routine Immunization SIAs Supplemental Immunization Activities STF State Task Force on Immunization UNICEF United Nations Children’s Fund VCM Volunteer Community Mobilizer VDPV2 Vaccine derived polio virus type 2 WHO World Health Organization WPV Wild polio virus 2 CONTENTS Executive Summary ………………………………………………………………………………………………………… 4 1.0 Introduction and context of the programme ……………………………………………………………. -

Nigeria – Complex Emergency JUNE 7, 2021
Fact Sheet #3 Fiscal Year (FY) 2021 Nigeria – Complex Emergency JUNE 7, 2021 SITUATION AT A GLANCE 206 8.7 2.9 308,000 12.8 MILLION MILLION MILLION MILLION Estimated Estimated Number of Estimated Estimated Projected Acutely Population People in Need in Number of IDPs Number of Food-Insecure w of Nigeria Northeast Nigeria in Nigeria Nigerian Refugees Population for 2021 in West Africa Lean Season UN – December 2020 UN – February 2021 UNHCR – February 2021 UNHCR – April 2021 CH – March 2021 Major OAG attacks on population centers in northeastern Nigeria—including Borno State’s Damasak town and Yobe State’s Geidam town—have displaced hundreds of thousands of people since late March. Intercommunal violence and OCG activity continue to drive displacement and exacerbate needs in northwest Nigeria. Approximately 12.8 million people will require emergency food assistance during the June-to-August lean season, representing a significant deterioration of food security in Nigeria compared with 2020. 1 TOTAL U.S. GOVERNMENT HUMANITARIAN FUNDING USAID/BHA $230,973,400 For the Nigeria Response in FY 2021 State/PRM2 $13,500,000 For complete funding breakdown with partners, see detailed chart on page 7 Total $244,473,400 1 USAID’s Bureau for Humanitarian Assistance (USAID/BHA) 2 U.S. Department of State Bureau for Population, Refugees, and Migration (State/PRM) 1 KEY DEVELOPMENTS Violence Drives Displacement and Constrains Access in the Northeast Organized armed group (OAG) attacks in Adamawa, Borno, and Yobe states have displaced more than 200,000 people since March and continue to exacerbate humanitarian needs and limit relief efforts, according to the UN. -

Drought Occurrences and Its Implications on the Households in Yobe State, Nigeria Jude Nwafor Eze
Eze Geoenvironmental Disasters (2018) 5:18 Geoenvironmental Disasters https://doi.org/10.1186/s40677-018-0111-7 RESEARCH Open Access Drought occurrences and its implications on the households in Yobe state, Nigeria Jude Nwafor Eze Abstract The study assesses the extent of droughts and its implications on the households in the study area. This is to highlight the need to integrate drought adaptation options into the government development plans. Strategies for drought adaptation options in the study area have often been made without experimental foundations placed on the extent of drought and its implications on the households. To achieve this, the study employed Normalized Rainfall Index (NRI) to determine the extent of droughts and its implications on the households, which has much to offer in terms of policy decisions. The study also utilized questionnaire administrated to 400 households to determine the annual income from different occupations that yielded more income to the people in the study area using one-way analysis of variance (ANOVA). The NRI shows that the study area was characterized by mild to severe drought events. The first (1986–1995) and third (2006–2017) decades experienced high incidences of droughts, while the second decade (1996–2005), witnessed the least incidences of droughts. The result of the economic activities of the households reveals that 65% of the total household respondents were involved in farming, while 35% were involved in non-farming activities as their major source of livelihood. The analysis of variance on the economic activities that generated more income to the households in Yobe State shows that farming activities provided more opportunities for income generation. -

Where Did Quantitative Metrics in Hausa and Other Chadic Songs Come From?1
1st International Conference on Endangered Languages Kano, 4-6 August 2014 WHERE DID QUANTITATIVE METRICS IN HAUSA AND OTHER CHADIC SONGS COME FROM?1 Russell G. Schuh UCLA The prosody of Hausa poetry and song is quantitative, that is, the rhythmic properties derive from syllable weight (heavy vs. light, or, alternatively, long vs. short). This is true for both formal written verse and for oral song ranging from the songs of professional singers to children’s songs. The prosody of Classical Arabic poetry is likewise quantitative. There is some controversy among Hausa scholars concerning the extent to which quantitative prosody in Hausa can be attributed to Arabic influence. Greenberg (1949) cited five characteristics of prosody that are shared by Arabic and Hausa as evidence that quantitative metrics in all Hausa poetry/song can be attributed to Arabic influence. This paper shows that folk songs of several minimally studied Chadic languages spoken in Yobe State, where there is no evidence of Arabic influence, share the five properties that Greenberg cited. The conclusion is that these features are essentially universal to the prosodic systems of languages in which syllable weight is salient. The prosodic systems of poetry/song in Arabic, Hausa, Yobe State Chadic languages, and more distantly related languages of the Afroasiatic phylum are all manifestation of the same underlying system. 1. Quantitative Poetic Meters The inherent rhythmic properties of metered poetry arise from two main sources, depending on the language: stress and quantity. One can illustrate these sources of rhythm with lines from English verse, which relies on syllabic stress, vs. -

English, Igbo, Yoruba Was Validated in December 2019 and in the Pro- and Pidgin English)
Contents Lists Of Acronyms........................................................................................................ 5 Foreword......................................................................................................................... 10 Executive Summary..................................................................................................... 12 Key Development Trends........................................................................................... 15 UN Nigeria 2019 at a glance..................................................................................... 17 2019 United Nations Sustainable Development Partnership Framework (UNSDPF) Results:...................................................................................................... 30 UNSDPF Result 1: All Nigerians enjoy good and inclusive governance and human rights in secure, resilient and peaceful communities......... 32 Outcome 1: Good governance and rule of law (Human Rights, Peace and Security) ................................................................................ 33 Outcome 2: Humanitarian response, peacebuilding and security .............................................................................................. 38 UNSDPF Result 2: Nigerians enjoy improved well-being through sustainable, equitable and quality basic services: .............................. 44 Outcome 3: Health, Nutrition and HIV/AIDS. ................................ 45 Outcome 4: Quality Learning and Skill Development ............... -

The Identity of the Catholic Church in Igboland, Nigeria
John Paul II Catholic University of Lublin Faculty of Theology Rev. Fr. Edwin Chukwudi Ezeokeke Index Number: 139970 THE IDENTITY OF THE CATHOLIC CHURCH IN IGBOLAND, NIGERIA Doctoral Thesis in Systematic Theology written under the supervision of Rev. Fr. Dr hab. Krzysztof Kaucha, prof. KUL Lublin 2018 1 DEDICATION This work is dedicated to the growth, strength and holiness of the Catholic Church in Igboland and the entire Universal Church. ACKNOWLEDGEMENT First of all, I give praise and glory to God Almighty, the creator and author of my being, essence and existence. I sincerely thank my Lord Bishop, Most Rev. Paulinus Chukwuemeka Ezeokafor for his paternal blessings, support and sponsorship. With deepest sentiments of gratitude, I thank tremendously my beloved parents, my siblings, my in-laws, friends and relatives for their great kindness and love. My unalloyed gratitude at this point goes to my brother priests here in Europe and America, Frs Anthony Ejeziem, Peter Okeke, Joseph Ibeanu and Paul Nwobi for their fraternal love and charity. My immeasurable gratitude goes to my distinguished and erudite moderator, Prof. Krzysztof Kaucha for his assiduousness, meticulosity and dedication in the moderation of this project. His passion for and profound lectures on Fundamental Theology offered me more stimulus towards developing a deeper interest in this area of ecclesiology. He guided me in formulating the theme and all through the work. I hugely appreciate his scholarly guidance, constant encouragement, thoughtful insights, valuable suggestions, critical observations and above all, his friendly approach. I also thank the Rector and all the Professors at John Paul II Catholic University, Lublin, Poland. -

Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria
Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria. May, 2015 Report on Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiasis in 19 States and the FCT, Nigeria. ii TABLE OF CONTENTS LIST OF FIGURES ...................................................................................................................................... v LIST OF PLATES ...................................................................................................................................... vii FOREWORD .............................................................................................................................................. x EXECUTIVE SUMMARY ........................................................................................................................... xii 1.0 BACKGROUND ................................................................................................................................... 1 1.1 Introduction ................................................................................................................................... 1 1.2 Objectives of the Mapping Project ................................................................................................ 2 1.3 Justification for the Survey ............................................................................................................ 2 2.0. MAPPING METHODOLOGY ..............................................................................................................