Biology and Potential Use of Chicken Bone Marrow-Derived Cells
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Adipose Derived Mesenchymal Stem Cell Differentiation Into Adipogenic and Osteogenic Stem Cells
vv ISSN: 2641-3000 DOI: https://dx.doi.org/10.17352/sscrt LIFE SCIENCES GROUP Hassan IH El Sayyad1*, Mohamed A Sobh2, Soad A Khalifa1 and Omnia KR Research Article 3 El-Sayyad Adipose Derived Mesenchymal Stem 1Zoology Department, Faculty of Science, Egypt 2Urology & Nephrology Center, Research Center, Egypt Cell Differentiation into Adipogenic 3Pediatric Mansoura University Hospital, Mansoura University, Egypt and Osteogenic Stem Cells Dates: Received: 08 December, 2016; Accepted: 23 December, 2016; Published: 29 December, 2016 *Corresponding author: Hassan IH El-Sayyad, Depart- Abstract ment of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt, Tel: 0020502254850; Objective: Lipoaspiration of human breast fats are important source of adipocyte stem cells E-mail: (hAMSCs) which play a great role in regenerative medicine. The present study illustrates its capability of its transformation and characterization of adipocyte, osteogenic or chondrogenic cells. https://www.peertechz.com Methods and results: The hAMSCs were positive for CD13, CD29, CD105 and CD90 and negative CD34 and CD 14. The hAMSCs were cultured in adipogenic or osteogenic culture for 4,7,14 & 21 days. Gene expression for adipogenic (PCR of leptin, peroxisome proliferator-activated receptor-γ and lipoprotein lipase) and osteogenic (osteocalcin) cells were carried out. Biochemical assessments of adipogenic (lipoprotein lipase enzyme and glycerol-3-phosphate dehydrogenase) and osteogenic (alkaline phosphatase, B-galactosidase and calcium content) markers. Also, light and transmission electron microscopic investigation of adipocyte stem cell culture were investigated at 4,7,14 & 21 days in both two models. Adipocyte derived from hAMSCs displayed fi broblastic morphology and confl uency at 7 days and fl at-shape with positive oil red staining at 14 &21 days. -

Pg 131 Chondroblast -> Chondrocyte (Lacunae) Firm Ground Substance
Figure 4.8g Connective tissues. Chondroblast ‐> Chondrocyte (Lacunae) Firm ground substance (chondroitin sulfate and water) Collagenous and elastic fibers (g) Cartilage: hyaline No BV or nerves Description: Amorphous but firm Perichondrium (dense irregular) matrix; collagen fibers form an imperceptible network; chondroblasts produce the matrix and when mature (chondrocytes) lie in lacunae. Function: Supports and reinforces; has resilient cushioning properties; resists compressive stress. Location: Forms most of the embryonic skeleton; covers the ends Chondrocyte of long bones in joint cavities; forms in lacuna costal cartilages of the ribs; cartilages of the nose, trachea, and larynx. Matrix Costal Photomicrograph: Hyaline cartilage from the cartilages trachea (750x). Thickness? Metabolism? Copyright © 2010 Pearson Education, Inc. Pg 131 Figure 6.1 The bones and cartilages of the human skeleton. Epiglottis Support Thyroid Larynx Smooth Cartilage in Cartilages in cartilage external ear nose surface Cricoid Trachea Articular Lung Cushions cartilage Cartilage of a joint Cartilage in Costal Intervertebral cartilage disc Respiratory tube cartilages in neck and thorax Pubic Bones of skeleton symphysis Meniscus (padlike Axial skeleton cartilage in Appendicular skeleton knee joint) Cartilages Articular cartilage of a joint Hyaline cartilages Elastic cartilages Fibrocartilages Pg 174 Copyright © 2010 Pearson Education, Inc. Figure 4.8g Connective tissues. (g) Cartilage: hyaline Description: Amorphous but firm matrix; collagen fibers form an imperceptible network; chondroblasts produce the matrix and when mature (chondrocytes) lie in lacunae. Function: Supports and reinforces; has resilient cushioning properties; resists compressive stress. Location: Forms most of the embryonic skeleton; covers the ends Chondrocyte of long bones in joint cavities; forms in lacuna costal cartilages of the ribs; cartilages of the nose, trachea, and larynx. -

A Regulator of Epiphyseal Plate Chondrocyte Proliferation, Hypertrophy, and Long Bone Growth
CHARACTERIZING AQP9: A REGULATOR OF EPIPHYSEAL PLATE CHONDROCYTE PROLIFERATION, HYPERTROPHY, AND LONG BONE GROWTH by Pontius Pu Tian Tang A thesis submitted in conformity with the requirements for the degree of Master of Science Institute of Medical Science University of Toronto © Copyright by Pontius Pu Tian Tang (2018) ii Abstract Characterizing Aqp9: a regulator of epiphyseal plate chondrocyte proliferation, hypertrophy, and long bone growth Pontius Pu Tian Tang Master of Science Institute of Medical Science University of Toronto 2018 Aquaporin-9 (AQP9) is a membrane channel protein suspected to regulate growth in the epiphyseal plate. As long bone defects often possess limited non-surgical options, novel factors underlying bone growth must be continuously explored to advance effective treatments. I hypothesized that Aqp9 is an important epiphyseal plate chondrocyte channel regulating the process of endochondral ossification. In this study, Aqp9 -/- mouse long bones compared to wildtype mouse long bones showed a neonatal hindlimb-specific acceleration of growth followed by reduced length in the juvenile age. Analysis of Aqp9 -/- epiphyseal plates and chondrocytes showed an early disposition for proliferation and aversion from hypertrophy, suggesting that Aqp9 may function similarly to genes such as Col10a1 and Mmp13. This study provides insight into chondrocyte membrane channel proteins and their regulation of the growing epiphyseal plate, demonstrating that Aqp9 may be a novel therapeutic target for the non-invasive intervention of leg length discrepancies. iii Acknowledgements I would like to take this opportunity to thank everyone who has helped me throughout my degree. Firstly, I would like to express my gratitude to my supervisor, Dr. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -
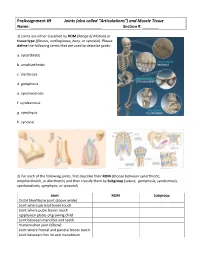
Preassignment #9 Joints (Also Called “Articulations”) and Muscle Tissue Name: Section #: __
PreAssignment #9 Joints (also called “Articulations”) and Muscle Tissue Name: _______________________________ Section #: _______ 1) Joints are either classified by ROM (Range of Motion) or tissue type (fibrous, cartilaginous, bony, or synovial). Please define the following terms that are used to describe joints: a. synarthrotic b. amphiarthrotic c. diarthrotic d. gomphosis e. synchondrosis f. syndesmosis g. symphysis h. synovial 2) For each of the following joints, first describe their ROM (choose between synarthrotic, amphiarthrotic, or diarthrotic) and then classify them by Subgroup (suture, gomphosis, syndesmosis, synchondrosis, symphysis, or synovial). Joint ROM Subgroup Distal tibiofibular joint (above ankle) Joint where parietal bones touch Joint where pubic bones touch Epiphyseal plates of growing child Joint between mandible and teeth Humeroulnar joint (elbow) Joint where frontal and parietal bones touch Joint between first rib and manubrium 3) Please label the parts of the generic synovial joint below. Use the terms: synovial membrane, ligament, periosteum, articular cartilage, synovial fluid, fibrous capsule, and joint capsule. Now, to see if you really understand joints, can you add labels for the diaphyseal cavity and epiphyseal line? 4) Synovial joints (or diarthroses) are usually classified as plane, hinge, saddle, ball-and-socket, or pivot joints. Note: I don’t list condyloid as a subgroup since it is really just a modified form of the hinges! For each of the following joints, please identify the classification to which they belong: a. humeroradial b. scapulohumeral c. interphalangeal d. atloaxis e. carpometacarpal #1 f. intercarpals g. proximal radioulnar h. sacroiliac 5) Movements at a joint are referred to as actions. For each of the following statements, tell me the proper action that is being used: a. -

Excision and Reimplantation of the Epiphyseal Cartilage of the Rabbit
[ 231 ] EXCISION AND REIMPLANTATION OF THE EPIPHYSEAL CARTILAGE OF THE RABBIT BY P. A. RING Charing Cross Hospital Medical School The postnatal growth of a typical long bone is associated with activity within the epiphyseal plates, and within the articular cartilage which surmounts the ends of the bone. The early enlargement of the epiphysis takes place at the expense of both epiphyseal and articular cartilage, as the illustrations of Gottesleben (1939) clearly show. Later enlargement of the epiphysis occurs solely on its articular aspect (Boerema, 1942). The epiphyseal cartilage thus shows an early bipolarity, although its contribution to shaft elongation is always greater than its contribution to epiphy- seal enlargement. With the formation of a terminal plate of bone upon its epiphyseal aspect, the epiphyseal cartilage loses this bipolarity, and its activities are restricted to new bone formation in the metaphyseal region. Payton (1933) has suggested that the region of this terminal plate is the site of absorption of epiphyseal bone. His deductions, however, from the madder-fed pig are circuitous and unsupported by the radiological evidence of Siegling (1941). The growth of the epiphysis itself has been fully discussed by Lacroix (1951). In the present investigation the polarity of the epiphyseal cartilage has been investigated by experiments in which the plate has been excised and replaced in its normal site, either orientated normally, or rotated through 1800 to bring the diaphyseal surface into contact with the epiphysis. The further growth of the bone, the extent, and the nature of the contribution from each end have been studied. METHOD Rabbits aged from 2 to 5 weeks were used. -

Connective Tissue
Study of Tissues Dr. A. Ebneshahidi ebneshahidi Tissues • Tissues are composed of cells similar in structure and specialized to perform a specific function for the body. • The human body is made of four general types of tissues. – Epithelial tissues – for lining body cavities, covering internal organs and large surfaces. – Connective tissues – for supporting and linking tissues or organs together; some are specialized to provide protection, to store fat, and even to provide circulatory function in the cardiovascular system. – Muscle tissues – for providing contraction and relaxation in the body surfaces, in the heart chambers , and in hollow organs such as blood vessels and the digestive tract. – Nerve tissue – for generating and transmitting electrical signals (nerve impulses ) in the brain, spinal cord, and nerves. ebneshahidi Epithelial tissues (Epithelium) • 1. Covering of body surfaces and internal organs, and lining of body cavities. • 2. Major tissue component of glands. • 3. Always has a free surface (exposed to an open space) and a basement membrane (usually anchored to a connective tissue). • 4. Lacks blood vessels , so nourishment comes from the underlying connective tissue by diffusion movement. • 5. Other unique characteristics: • a. Reproduce rapidly. • b. Cells in epithelial tissues are often attached to one another by desmosomes which allow the tissue to serve as an excellent protective layer. ebneshahidi • c. The name is derived from the number of layer of cells ("simple" means a single layer while "stratified " means multiple layers ) and the shape of cells ("squamous" means flattened , "cuboidal" means cube – shaped ,and "columnar" means elongated ). ebneshahidi 1. Simple squamous epithelium – a single layer of thin , flattened cells. -

Chondrogenesis, Studied with the Electron Microscope
CHONDROGENESIS, STUDIED WITH THE ELECTRON MICROSCOPE GABRIEL C. GODMAN, M.1)., and KEITH R. PORTER, Ph.I). From The Rockefeller Institute. Dr. Godman's present address is Department of Microbiology, Columbia University, New York ABSTRACT The role of the cells in the fabrication of a connective tissue matrix, and the structural modifications which accompany cytodifferentiation have been investigated in developing epiphyseal cartilage of fetal rat by means of electron microscopy. Differentiation of the pre- chondral mesenchymal cells to chondroblasts is marked by the acquisition of an extensive endoplasmic reticulum, enlargement and concentration of the Golgi apparatus, the ap- pearance of membrane-bounded cytoplasmic inclusions, and the formation of specialized foci of increased density in the cell cortex. These modifications are related to the secretion of the cartilage matrix. The matrix of young hyaline cartilage consists of groups of rela- tively short, straight, banded collagen fibrils of 10 to 20 m# and a dense granular component embedded in an amorphous ground substance of moderate electron density. It is postu- lated that the first phase of fibrillogenesis takes place at the cell cortex in dense bands or striae within the ectoplasm subjacent to the cell membrane. These can be resolved into sheaves of "primary" fibrils of about 7 to 10 m#. They are supposedly shed (by excortica- tion) into the matrix space between the separating chondroblasts, where they may serve as "cores" of the definitive matrix fibrils. The diameter of the fibrils may subsequently increase up to threefold, presumably by incorporation of "soluble" or tropocollagen units from the ground substance. The chondroblast also discharges into the matrix the electron- dense amorphous or granular contents of vesicles derived from the Golgi apparatus, and the mixed contents of large vacuoles or blebs bounded by distinctive double membranes. -

Aandp1ch07lecture.Pdf
Chapter 07 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • In this chapter we will cover: – Bone tissue composition – How bone functions, develops, and grows – How bone metabolism is regulated and some of its disorders 7-2 Introduction • Bones and teeth are the most durable remains of a once-living body • Living skeleton is made of dynamic tissues, full of cells, permeated with nerves and blood vessels • Continually remodels itself and interacts with other organ systems of the body • Osteology is the study of bone 7-3 Tissues and Organs of the Skeletal System • Expected Learning Outcomes – Name the tissues and organs that compose the skeletal system. – State several functions of the skeletal system. – Distinguish between bones as a tissue and as an organ. – Describe the four types of bones classified by shape. – Describe the general features of a long bone and a flat bone. 7-4 Tissues and Organs of the Skeletal System • Skeletal system—composed of bones, cartilages, and ligaments – Cartilage—forerunner of most bones • Covers many joint surfaces of mature bone – Ligaments—hold bones together at joints – Tendons—attach muscle to bone 7-5 Functions of the Skeleton • Support—limb bones and vertebrae support body; jaw bones support teeth; some bones support viscera • Protection—of brain, spinal cord, heart, lungs, and more • Movement—limb movements, breathing, and other -

A Morphological and Histological Study of the Interface Between Bone and the Attachments of Quadriceps Tendon and Patellar Ligament
A morphological and histological study of the interface between bone and the attachments of quadriceps tendon and patellar ligament Motabagani , Mohammed A. and Abdel Meguid, Eiman M. Department of Anatomy, King Faisal University, Dammam, Saudi Arabia. Abstract Knee ligaments anatomy has been recently the focus of research because of the frequent need for reconstructive arthroplastic surgery in this area. At present, the implantation of patellar tendon is regarded as a golden standard for anterior cruciate ligament reconstruction surgery. The present study was undertaken to investigate the anterior cruciate ligament and patellar tendon morphologically, histologically and radiologically to demonstrate the use of patellar tendon in autograft reconstructive knee arthoplasty. Morphology at ligament and tendon insertions were studied to establish a background for subsequent studies of insertion altered by disease and to gain impression of function based on structural relations. Morphology of quadriceps tendon insertion with special emphasis on the shape of soft tissue/bone interface and thickness of calcified fibrocartilage were studied. Specimens were taken from 50 rabbit's patellae and 40 cadaveric patellae. Using MRI taken from 50 living human subjects, the actual length relationship of knee ligaments were measured. The morphology of the interface between patellar tendon and bone was described in relation to the mechanical properties and the response of bone to stress. A description of the transformation zones that occurs in the structure of patellar tendon and ligament as they insert into the bone was demonstrated. The differences between the quantities and distribution of uncalcified fibrocartilage at the attachment of quadriceps and the origin and insertion of patellar ligament were reported. -

Chapter 6: Bones & Skeletal Tissue
Chapter 6: Bones & Skeletal Tissue I. Skeletal Cartilage A. Basic Structure, Types and Location 1. Skeletal cartilages are made from cartilage, surrounded by a layer of dense irregular connective tissue called perichondrium 2. Hyaline cartilage is most abundant and includes: articular, costal, respiratory and nasal cartilage 3. Elastic Cartilages are most flexible and are located in the external ear and the epiglottis of the larnyx 4. Fibrocartilage is located in the areas where large amounts of pressure are exerted or stretch occurs ( knees and intervertbral discs) B. Growth of Cartilage 1. Appositional growth results in outward expansion due to the production of cartilage matrix on the outer face of the tissue 2. Interstitial growth is the results in the expansion from within the cartilage matrix due to the division of the lacunae bound chondrocytes II. Classification of Bone A. Divisions of the skeleton 1. Axial: Includes the rib cage and vertebral column, 2. Appendicular: Includes the bones of the upper limb, lower limb and the girdles that attach them to the axial skeleton B. Shape 1. Long bones are longer than they are wide, have a definite shaft two ends and consist of all the limb bones except the patella, carpals and tarsals 2. Short bones are somewhat cube shaped and include the the carpals, tarsals and patellas 3. Flat bones are thin, flattened, and often curve that include skull bones, sternum, scapulae and ribs 4. Irregular bones have complicated shapes the do not fit into any other class such as the vertebrae and coxae III. Function of Bones A.