Open Gavazzi Thesis Final SHC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Regulator of Epiphyseal Plate Chondrocyte Proliferation, Hypertrophy, and Long Bone Growth
CHARACTERIZING AQP9: A REGULATOR OF EPIPHYSEAL PLATE CHONDROCYTE PROLIFERATION, HYPERTROPHY, AND LONG BONE GROWTH by Pontius Pu Tian Tang A thesis submitted in conformity with the requirements for the degree of Master of Science Institute of Medical Science University of Toronto © Copyright by Pontius Pu Tian Tang (2018) ii Abstract Characterizing Aqp9: a regulator of epiphyseal plate chondrocyte proliferation, hypertrophy, and long bone growth Pontius Pu Tian Tang Master of Science Institute of Medical Science University of Toronto 2018 Aquaporin-9 (AQP9) is a membrane channel protein suspected to regulate growth in the epiphyseal plate. As long bone defects often possess limited non-surgical options, novel factors underlying bone growth must be continuously explored to advance effective treatments. I hypothesized that Aqp9 is an important epiphyseal plate chondrocyte channel regulating the process of endochondral ossification. In this study, Aqp9 -/- mouse long bones compared to wildtype mouse long bones showed a neonatal hindlimb-specific acceleration of growth followed by reduced length in the juvenile age. Analysis of Aqp9 -/- epiphyseal plates and chondrocytes showed an early disposition for proliferation and aversion from hypertrophy, suggesting that Aqp9 may function similarly to genes such as Col10a1 and Mmp13. This study provides insight into chondrocyte membrane channel proteins and their regulation of the growing epiphyseal plate, demonstrating that Aqp9 may be a novel therapeutic target for the non-invasive intervention of leg length discrepancies. iii Acknowledgements I would like to take this opportunity to thank everyone who has helped me throughout my degree. Firstly, I would like to express my gratitude to my supervisor, Dr. -
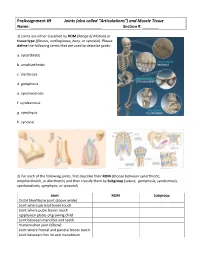
Preassignment #9 Joints (Also Called “Articulations”) and Muscle Tissue Name: Section #: __
PreAssignment #9 Joints (also called “Articulations”) and Muscle Tissue Name: _______________________________ Section #: _______ 1) Joints are either classified by ROM (Range of Motion) or tissue type (fibrous, cartilaginous, bony, or synovial). Please define the following terms that are used to describe joints: a. synarthrotic b. amphiarthrotic c. diarthrotic d. gomphosis e. synchondrosis f. syndesmosis g. symphysis h. synovial 2) For each of the following joints, first describe their ROM (choose between synarthrotic, amphiarthrotic, or diarthrotic) and then classify them by Subgroup (suture, gomphosis, syndesmosis, synchondrosis, symphysis, or synovial). Joint ROM Subgroup Distal tibiofibular joint (above ankle) Joint where parietal bones touch Joint where pubic bones touch Epiphyseal plates of growing child Joint between mandible and teeth Humeroulnar joint (elbow) Joint where frontal and parietal bones touch Joint between first rib and manubrium 3) Please label the parts of the generic synovial joint below. Use the terms: synovial membrane, ligament, periosteum, articular cartilage, synovial fluid, fibrous capsule, and joint capsule. Now, to see if you really understand joints, can you add labels for the diaphyseal cavity and epiphyseal line? 4) Synovial joints (or diarthroses) are usually classified as plane, hinge, saddle, ball-and-socket, or pivot joints. Note: I don’t list condyloid as a subgroup since it is really just a modified form of the hinges! For each of the following joints, please identify the classification to which they belong: a. humeroradial b. scapulohumeral c. interphalangeal d. atloaxis e. carpometacarpal #1 f. intercarpals g. proximal radioulnar h. sacroiliac 5) Movements at a joint are referred to as actions. For each of the following statements, tell me the proper action that is being used: a. -

Excision and Reimplantation of the Epiphyseal Cartilage of the Rabbit
[ 231 ] EXCISION AND REIMPLANTATION OF THE EPIPHYSEAL CARTILAGE OF THE RABBIT BY P. A. RING Charing Cross Hospital Medical School The postnatal growth of a typical long bone is associated with activity within the epiphyseal plates, and within the articular cartilage which surmounts the ends of the bone. The early enlargement of the epiphysis takes place at the expense of both epiphyseal and articular cartilage, as the illustrations of Gottesleben (1939) clearly show. Later enlargement of the epiphysis occurs solely on its articular aspect (Boerema, 1942). The epiphyseal cartilage thus shows an early bipolarity, although its contribution to shaft elongation is always greater than its contribution to epiphy- seal enlargement. With the formation of a terminal plate of bone upon its epiphyseal aspect, the epiphyseal cartilage loses this bipolarity, and its activities are restricted to new bone formation in the metaphyseal region. Payton (1933) has suggested that the region of this terminal plate is the site of absorption of epiphyseal bone. His deductions, however, from the madder-fed pig are circuitous and unsupported by the radiological evidence of Siegling (1941). The growth of the epiphysis itself has been fully discussed by Lacroix (1951). In the present investigation the polarity of the epiphyseal cartilage has been investigated by experiments in which the plate has been excised and replaced in its normal site, either orientated normally, or rotated through 1800 to bring the diaphyseal surface into contact with the epiphysis. The further growth of the bone, the extent, and the nature of the contribution from each end have been studied. METHOD Rabbits aged from 2 to 5 weeks were used. -

Aandp1ch07lecture.Pdf
Chapter 07 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • In this chapter we will cover: – Bone tissue composition – How bone functions, develops, and grows – How bone metabolism is regulated and some of its disorders 7-2 Introduction • Bones and teeth are the most durable remains of a once-living body • Living skeleton is made of dynamic tissues, full of cells, permeated with nerves and blood vessels • Continually remodels itself and interacts with other organ systems of the body • Osteology is the study of bone 7-3 Tissues and Organs of the Skeletal System • Expected Learning Outcomes – Name the tissues and organs that compose the skeletal system. – State several functions of the skeletal system. – Distinguish between bones as a tissue and as an organ. – Describe the four types of bones classified by shape. – Describe the general features of a long bone and a flat bone. 7-4 Tissues and Organs of the Skeletal System • Skeletal system—composed of bones, cartilages, and ligaments – Cartilage—forerunner of most bones • Covers many joint surfaces of mature bone – Ligaments—hold bones together at joints – Tendons—attach muscle to bone 7-5 Functions of the Skeleton • Support—limb bones and vertebrae support body; jaw bones support teeth; some bones support viscera • Protection—of brain, spinal cord, heart, lungs, and more • Movement—limb movements, breathing, and other -

Chapter 6: Bones & Skeletal Tissue
Chapter 6: Bones & Skeletal Tissue I. Skeletal Cartilage A. Basic Structure, Types and Location 1. Skeletal cartilages are made from cartilage, surrounded by a layer of dense irregular connective tissue called perichondrium 2. Hyaline cartilage is most abundant and includes: articular, costal, respiratory and nasal cartilage 3. Elastic Cartilages are most flexible and are located in the external ear and the epiglottis of the larnyx 4. Fibrocartilage is located in the areas where large amounts of pressure are exerted or stretch occurs ( knees and intervertbral discs) B. Growth of Cartilage 1. Appositional growth results in outward expansion due to the production of cartilage matrix on the outer face of the tissue 2. Interstitial growth is the results in the expansion from within the cartilage matrix due to the division of the lacunae bound chondrocytes II. Classification of Bone A. Divisions of the skeleton 1. Axial: Includes the rib cage and vertebral column, 2. Appendicular: Includes the bones of the upper limb, lower limb and the girdles that attach them to the axial skeleton B. Shape 1. Long bones are longer than they are wide, have a definite shaft two ends and consist of all the limb bones except the patella, carpals and tarsals 2. Short bones are somewhat cube shaped and include the the carpals, tarsals and patellas 3. Flat bones are thin, flattened, and often curve that include skull bones, sternum, scapulae and ribs 4. Irregular bones have complicated shapes the do not fit into any other class such as the vertebrae and coxae III. Function of Bones A. -

4 Anat 35 Articulations
Human Anatomy Unit 1 ARTICULATIONS In Anatomy Today Classification of Joints • Criteria – How bones are joined together – Degree of mobility • Minimum components – 2 articulating bones – Intervening tissue • Fibrous CT or cartilage • Categories – Synarthroses – no movement – Amphiarthrosis – slight movement – Diarthrosis – freely movable Synarthrosis • Immovable articulation • Types – Sutures – Schindylesis – Gomphosis – Synchondrosis Synarthrosis Sutures • Found only in skull • Immovable articulation • Flat bones joined by thin layer of fibrous CT • Types – Serrate – Squamous (lap) – Plane Synarthrosis Sutures • Serrate • Serrated edges of bone interlock • Two portions of frontal bones • Squamous (lap) • Overlapping beveled margins forms smooth line • Temporal and parietal bones • Plane • Joint formed by straight, nonoverlapping edges • Palatine process of maxillae Synarthrosis Schindylesis • Immovable articulation • Thin plate of bone into cleft or fissure in a separation of the laminae in another bone • Articulation of sphenoid bone and perpendicular plate of ethmoid bone with vomer Synarthrosis Gomphosis • Immovable articulation • Conical process into a socket • Articulation of teeth with alveoli of maxillary bone • Periodontal ligament = fibrous CT Synarthrosis Synchondrosis • Cartilagenous joints – Ribs joined to sternum by hyaline cartilage • Synostoses – When joint ossifies – Epiphyseal plate becomes epiphyseal line Amphiarthrosis • Slightly moveable articulation • Articulating bones connected in one of two ways: – By broad flattened -

Estimation of Biological Age by Epiphyseal Union Jonathon Lewis Western Oregon University, [email protected]
Western Oregon University Digital Commons@WOU Academic Excellence Showcase Proceedings Student Scholarship 2012-05-31 Estimation of Biological Age by Epiphyseal Union Jonathon Lewis Western Oregon University, [email protected] Follow this and additional works at: https://digitalcommons.wou.edu/aes Part of the Anthropology Commons Citation Lewis, Jonathon. "Estimation of Biological Age by Epiphyseal Union." Poster presented at the Academic Excellence Showcase, Western Oregon University, May 31, 2012. This Poster is brought to you for free and open access by the Student Scholarship at Digital Commons@WOU. It has been accepted for inclusion in Academic Excellence Showcase Proceedings by an authorized administrator of Digital Commons@WOU. For more information, please contact [email protected], [email protected], [email protected]. Estimation of Biological Age by Epiphyseal Union Jonathon Lewis Western Oregon University Abstract Methods Figure 2. Sacral epiphyseal line scored a 2.5 Epiphyseal union is the secondary ossification of bone in Upon gaining access to the postcranial remains, a full and juveniles and primary means of estimating age of subadult thorough inventory was conducted. Following protocols post-cranial remains. First an inventory was conducted of developed by Lieverse (2006) this inventory accomplished skeletal remains from a single individual, accounting for the many goals. First, the assessment accounted for exactly skeletal condition as well as the presence or absence of which bones of the skeleton were present or absent. bones. Then, using previously developed standards, the Figure 6. Right humeral epiphyseal line Figure 7. Lumbar 4th Second, the assessment took into consideration the scored a 2.5 vertebra epiphyseal line estimation of age was assessed through a system of scoring degree of completeness of each bone present. -

Treatment of Avascular Necrosis of the Femoral Head by Hepatocyte Growth Factor-Transgenic Bone Marrow Stromal Stem Cells
Gene Therapy (2008) 15, 1523–1535 & 2008 Macmillan Publishers Limited All rights reserved 0969-7128/08 $32.00 www.nature.com/gt ORIGINAL ARTICLE Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells QWen1,LMa1, Y-P Chen2, L Yang2, W Luo1 and X-N Wang3 1Institute of Molecular Immunology, Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Imageology, Nanfang Hospital, Guangzhou, People’s Republic of China and 3School of Biosciences and Bioengineering, South China University of Technology, Guangzhou, People’s Republic of China The treatment of hormone-induced early-stage avascular pathological sections. A regular arrangement of trabeculae necrosis of the femoral head (ANFH) with transplantation of and obvious bone regeneration were observed in the hepatocyte growth factor (HGF)-transgenic bone marrow animals receiving transplanted transgenic BMSCs with stromal stem cells (BMSCs) was examined. A rabbit model of FG. Newly generated capillaries were visible on the hormone-induced early ANFH was first established. BMSCs bone plates of the trabeculae, and the bone marrow was were transplanted by core decompression under the rich in hematopoietic tissue. These results demonstrate guidance of computed tomography (CT). A supportive that the combination of core decompression and trans- fibrinogen drug delivery mixture (FG) was tested for plantation of HGF transgenic autologous BMSCs enhanced mechanical enhancement of stem cell delivery. Therapeutic blood vessel regeneration and bone reconstruction in efficacy was evaluated by CT, magnetic resonance imaging the ANFH model. This study provides experimental data that (MRI), CT perfusion imaging, ink artery infusion angiography, motivate possible clinical use of this therapeutic strategy. -

FIPAT-TA2-Part-2.Pdf
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART II Contents: Systemata musculoskeletalia Musculoskeletal systems Caput II: Ossa Chapter 2: Bones Caput III: Juncturae Chapter 3: Joints Caput IV: Systema musculare Chapter 4: Muscular system Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput II: OSSA Chapter 2: BONES Latin term Latin synonym UK English US English English synonym Other 351 Systemata Musculoskeletal Musculoskeletal musculoskeletalia systems systems -

Bone Marrow Stimulation of the Medial Femoral Condyle Produces Inferior Cartilage and Bone Repair Compared to the Trochlea in a Rabbit Surgical Model
Bone Marrow Stimulation of the Medial Femoral Condyle Produces Inferior Cartilage and Bone Repair Compared to the Trochlea in a Rabbit Surgical Model Hongmei Chen,1 Anik Chevrier,1 Caroline D. Hoemann,1 Jun Sun,2 Genevieve Picard,1 Michael D. Buschmann1 1Department of Chemical Engineering and Institute of Biomedical Engineering, E´cole Polytechnique de Montre´al, PO Box 6079, Station Centre- ville, Montre´al, Quebec, Canada H3C 3A7, 2Piramal Healthcare (Canada), BioSyntech Canada Inc., Laval, Quebec, Canada Received 15 September 2012; accepted 6 June 2013 Published online in Wiley Online Library (wileyonlinelibrary.com). DOI 10.1002/jor.22422 ABSTRACT: The influence of the location of cartilage lesions on cartilage repair outcome is incompletely understood. This study compared cartilage and bone repair in medial femoral condylar (MFC) versus femoral trochlear (TR) defects 3 months after bone marrow stimulation in mature rabbits. Intact femurs from adult rabbits served as controls. Results from quantitative histomorphom- etry and histological scoring showed that bone marrow stimulation produced inferior soft tissue repair in MFC versus TR defects, as indicated by significantly lower % Fill (p ¼ 0.03), a significant increase in collagen type I immunostaining (p < 0.00001) and lower O’Driscoll scores (p < 0.05). 3D micro-CT analysis showed that repaired TR defects regained normal un-operated values of bone volume fraction, trabecular thickness, and trabecular number, whereas in MFC defects the repaired bone architecture appeared immature and less dense compared to intact un-operated MFC controls (p < 0.0001). Severe medial meniscal damage was found in 28% of operated animals and was strongly correlated with (i) low cartilage defect fill, (ii) incomplete bone repair in MFC, and (iii) with a more posterior defect placement in the weight-bearing region. -

Age and Sex Estimation from the Human Clavicle: an Investigation of Traditional and Novel Methods
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: AGE AND SEX ESTIMATION FROM THE HUMAN CLAVICLE: AN INVESTIGATION OF TRADITIONAL AND NOVEL METHODS Author: Natalie Renee Shirley Document No.: 227930 Date Received: August 2009 Award Number: 2007-DN-BX-0004 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant final report available electronically in addition to traditional paper copies. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. AGE AND SEX ESTIMATION FROM THE HUMAN CLAVICLE: AN INVESTIGATION OF TRADITIONAL AND NOVEL METHODS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Natalie Renee Shirley May 2009 i This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. I dedicate this dissertation to Linda Finley, because without her none of this would have ever happened. -

Chapter 6 Skeletal System • the Structural Framework That Gives the Body Shape & Provides Protection for Internal Organs & Soft Tissue
Chapter 6 Skeletal System • The structural framework that gives the body shape & provides protection for internal organs & soft tissue. • Skeleton derived from Greek: meaning dried • Dynamic, living tissues that are capable of growth, adaptation to stress, & undergoing repair after injury AP1 Chapter 6 1 Chapter 6 Outline I. Fxn of the Skeletal System II. Cartilage III. Bone Histology IV. Bone Anatomy V. Bone Development VI. Bone Growth VII. Bone remodeling VIII. Bone repair IX. Calcium Homeostasis X. FX of aging on Skeletal System AP1 Chapter 6 2 I. Fxns of the Skeletal System 4 components make-up SS 1. Bones 2. Cartilage 3. Tendons 4. Ligaments AP1 Chapter 6 3 5 fxns of SS 1. Support – Bone: rigid, strong well suited for bearing weight & major supporting tissue for body – Cartilage: firm yet flexible support – Ligaments: strong bands of fibrous CT that hold bones together 2. Protection – Forms a hard shell protecting organs within 3. Movement – Muscles attach to tendons wh/attach to bone and use the joints of those bones as pivot points. Hyaline cartilage also covers bones ends and allows for smoother movement. Ligaments help prevent XSV movement 4. Storage – Mineral Storage (Ca PO4) can be deposited and extracted when needed – Yellow Bone Marrow: stored in bone cavities and can be extracted for energy use 5. Blood cell production – Red Bone Marrow: gives rise to blood cells & platelets AP1 Chapter 6 4 II. Cartilage AP1 Chapter 6 5 II. Cartilage Figure 6.1 Pg 174 • 3 types of cartilage: – Elastic, Fibrocartilage, & hyaline • Hyaline (HC) is most closely associated w/bone: Most bones develop from it Bone growth in length involves HC Bone repair involves prod’n of HC followed by bone replacement AP1 Chapter 6 6 II.