Introduction to Nuclear Physics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

The R-Process Nucleosynthesis and Related Challenges
EPJ Web of Conferences 165, 01025 (2017) DOI: 10.1051/epjconf/201716501025 NPA8 2017 The r-process nucleosynthesis and related challenges Stephane Goriely1,, Andreas Bauswein2, Hans-Thomas Janka3, Oliver Just4, and Else Pllumbi3 1Institut d’Astronomie et d’Astrophysique, Université Libre de Bruxelles, CP 226, 1050 Brussels, Belgium 2Heidelberger Institut fr¨ Theoretische Studien, Schloss-Wolfsbrunnenweg 35, 69118 Heidelberg, Germany 3Max-Planck-Institut für Astrophysik, Postfach 1317, 85741 Garching, Germany 4Astrophysical Big Bang Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198, Japan Abstract. The rapid neutron-capture process, or r-process, is known to be of fundamental importance for explaining the origin of approximately half of the A > 60 stable nuclei observed in nature. Recently, special attention has been paid to neutron star (NS) mergers following the confirmation by hydrodynamic simulations that a non-negligible amount of matter can be ejected and by nucleosynthesis calculations combined with the predicted astrophysical event rate that such a site can account for the majority of r-material in our Galaxy. We show here that the combined contribution of both the dynamical (prompt) ejecta expelled during binary NS or NS-black hole (BH) mergers and the neutrino and viscously driven outflows generated during the post-merger remnant evolution of relic BH-torus systems can lead to the production of r-process elements from mass number A > 90 up to actinides. The corresponding abundance distribution is found to reproduce the∼ solar distribution extremely well. It can also account for the elemental distributions observed in low-metallicity stars. However, major uncertainties still affect our under- standing of the composition of the ejected matter. -
Pair Energy of Proton and Neutron in Atomic Nuclei
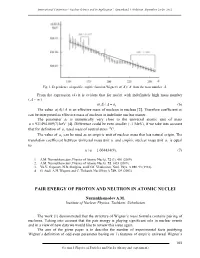
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Photofission Cross Sections of 238U and 235U from 5.0 Mev to 8.0 Mev Robert Andrew Anderl Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV Robert Andrew Anderl Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons, and the Oil, Gas, and Energy Commons Recommended Citation Anderl, Robert Andrew, "Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV " (1972). Retrospective Theses and Dissertations. 4715. https://lib.dr.iastate.edu/rtd/4715 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction, 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity, 2. -

1 Lecture Notes in Nuclear Structure Physics B. Alex Brown November
1 Lecture Notes in Nuclear Structure Physics B. Alex Brown November 2005 National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy Michigan State University, E. Lansing, MI 48824 CONTENTS 2 Contents 1 Nuclear masses 6 1.1 Masses and binding energies . 6 1.2 Q valuesandseparationenergies . 10 1.3 Theliquid-dropmodel .......................... 18 2 Rms charge radii 25 3 Charge densities and form factors 31 4 Overview of nuclear decays 40 4.1 Decaywidthsandlifetimes. 41 4.2 Alphaandclusterdecay ......................... 42 4.3 Betadecay................................. 51 4.3.1 BetadecayQvalues ....................... 52 4.3.2 Allowedbetadecay ........................ 53 4.3.3 Phase-space for allowed beta decay . 57 4.3.4 Weak-interaction coupling constants . 59 4.3.5 Doublebetadecay ........................ 59 4.4 Gammadecay............................... 61 4.4.1 Reduced transition probabilities for gamma decay . .... 62 4.4.2 Weisskopf units for gamma decay . 65 5 The Fermi gas model 68 6 Overview of the nuclear shell model 71 7 The one-body potential 77 7.1 Generalproperties ............................ 77 7.2 Theharmonic-oscillatorpotential . ... 79 7.3 Separation of intrinsic and center-of-mass motion . ....... 81 7.3.1 Thekineticenergy ........................ 81 7.3.2 Theharmonic-oscillator . 83 8 The Woods-Saxon potential 87 8.1 Generalform ............................... 87 8.2 Computer program for the Woods-Saxon potential . .... 91 8.2.1 Exampleforboundstates . 92 8.2.2 Changingthepotentialparameters . 93 8.2.3 Widthofanunboundstateresonance . 94 8.2.4 Width of an unbound state resonance at a fixed energy . 95 9 The general many-body problem for fermions 97 CONTENTS 3 10 Conserved quantum numbers 101 10.1 Angularmomentum. 101 10.2Parity .................................. -

14. Structure of Nuclei Particle and Nuclear Physics
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14. Structure of Nuclei 2 Magic Numbers Magic Numbers = 2; 8; 20; 28; 50; 82; 126... Nuclei with a magic number of Z and/or N are particularly stable, e.g. Binding energy per nucleon is large for magic numbers Doubly magic nuclei are especially stable. Dr. Tina Potter 14. Structure of Nuclei 3 Magic Numbers Other notable behaviour includes Greater abundance of isotopes and isotones for magic numbers e.g. Z = 20 has6 stable isotopes (average=2) Z = 50 has 10 stable isotopes (average=4) Odd A nuclei have small quadrupole moments when magic First excited states for magic nuclei higher than neighbours Large energy release in α, β decay when the daughter nucleus is magic Spontaneous neutron emitters have N = magic + 1 Nuclear radius shows only small change with Z, N at magic numbers. etc... etc... Dr. Tina Potter 14. Structure of Nuclei 4 Magic Numbers Analogy with atomic behaviour as electron shells fill. Atomic case - reminder Electrons move independently in central potential V (r) ∼ 1=r (Coulomb field of nucleus). Shells filled progressively according to Pauli exclusion principle. Chemical properties of an atom defined by valence (unpaired) electrons. Energy levels can be obtained (to first order) by solving Schr¨odinger equation for central potential. 1 E = n = principle quantum number n n2 Shell closure gives noble gas atoms. Are magic nuclei analogous to the noble gas atoms? Dr. -

Production and Properties Towards the Island of Stability
This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Author(s): Leino, Matti Title: Production and properties towards the island of stability Year: 2016 Version: Please cite the original version: Leino, M. (2016). Production and properties towards the island of stability. In D. Rudolph (Ed.), Nobel Symposium NS 160 - Chemistry and Physics of Heavy and Superheavy Elements (Article 01002). EDP Sciences. EPJ Web of Conferences, 131. https://doi.org/10.1051/epjconf/201613101002 All material supplied via JYX is protected by copyright and other intellectual property rights, and duplication or sale of all or part of any of the repository collections is not permitted, except that material may be duplicated by you for your research use or educational purposes in electronic or print form. You must obtain permission for any other use. Electronic or print copies may not be offered, whether for sale or otherwise to anyone who is not an authorised user. EPJ Web of Conferences 131, 01002 (2016) DOI: 10.1051/epjconf/201613101002 Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements Production and properties towards the island of stability Matti Leino Department of Physics, University of Jyväskylä, PO Box 35, 40014 University of Jyväskylä, Finland Abstract. The structure of the nuclei of the heaviest elements is discussed with emphasis on single-particle properties as determined by decay and in- beam spectroscopy. The basic features of production of these nuclei using fusion evaporation reactions will also be discussed. 1. Introduction In this short review, some examples of nuclear structure physics and experimental methods relevant for the study of the heaviest elements will be presented. -

Lecture 3: Nuclear Structure 1 • Why Structure? • the Nuclear Potential • Schematic Shell Model
Lecture 3: Nuclear Structure 1 • Why structure? • The nuclear potential • Schematic shell model Lecture 3: Ohio University PHYS7501, Fall 2017, Z. Meisel ([email protected]) Empirically, several striking trends related to Z,N. e.g. B.A. Brown, Lecture Notes in Nuclear Structure Physics, 2005. M.A. Preston, Physics of the Nucleus (1962) Q B.A. Brown, Lecture Notes in Nuclear Structure Physics, 2005. Adapted from B.A. Brown, C.R. Bronk, & P.E. Hodgson, J. Phys. G (1984) P. Möller et al. ADNDT (2016) Meisel & George, IJMS (2013) A. Cameron, Proc. Astron. Soc. Pac. (1957) First magic number evidence compilation 2 by M. Göppert-Mayer Phys. Rev. 1948 …reminiscent of atomic structure B.A. Brown, Lecture Notes in Nuclear Structure Physics, 2005. hyperphysics hyperphysics 54 3 Shell Structure Atomic Nuclear •Central potential (Coulomb) generated by nucleus •No central object …but each nucleon is interacted on by the other A-1 nucleons and they’re relatively compact together •Electrons are essentially non-interacting •Nucleons interact very strongly …but if nucleons in nucleus were to scatter, Pauli blocking prevents them from scattering into filled orbitals. Scattering into higher-E orbitals is unlikely. i.e. there is no “weak interaction paradox” •Solve the Schrödinger equation for the Coulomb •Can also solve the Schrödinger equation for energy potential and find characteristic (energy levels) shells: levels (shells) …but obviously must be a different shells at 2, 10, 18, 36, 54, 86 potential: shells at 2, 8, 20, 28, 50, 82, 126 …you might be discouraged by points 1 and 2 above, but, remember: If it’s stupid but it works, it isn’t stupid. -

Prospects for Measurements with Strange Hadrons at Lhcb
Prospects for measurements with strange hadrons at LHCb A. A. Alves Junior1, M. O. Bettler2, A. Brea Rodr´ıguez1, A. Casais Vidal1, V. Chobanova1, X. Cid Vidal1, A. Contu3, G. D'Ambrosio4, J. Dalseno1, F. Dettori5, V.V. Gligorov6, G. Graziani7, D. Guadagnoli8, T. Kitahara9;10, C. Lazzeroni11, M. Lucio Mart´ınez1, M. Moulson12, C. Mar´ınBenito13, J. Mart´ınCamalich14;15, D. Mart´ınezSantos1, J. Prisciandaro 1, A. Puig Navarro16, M. Ramos Pernas1, V. Renaudin13, A. Sergi11, K. A. Zarebski11 1Instituto Galego de F´ısica de Altas Enerx´ıas(IGFAE), Santiago de Compostela, Spain 2Cavendish Laboratory, University of Cambridge, Cambridge, United Kingdom 3INFN Sezione di Cagliari, Cagliari, Italy 4INFN Sezione di Napoli, Napoli, Italy 5Oliver Lodge Laboratory, University of Liverpool, Liverpool, United Kingdom, now at Universit`adegli Studi di Cagliari, Cagliari, Italy 6LPNHE, Sorbonne Universit´e,Universit´eParis Diderot, CNRS/IN2P3, Paris, France 7INFN Sezione di Firenze, Firenze, Italy 8Laboratoire d'Annecy-le-Vieux de Physique Th´eorique , Annecy Cedex, France 9Institute for Theoretical Particle Physics (TTP), Karlsruhe Institute of Technology, Kalsruhe, Germany 10Institute for Nuclear Physics (IKP), Karlsruhe Institute of Technology, Kalsruhe, Germany 11School of Physics and Astronomy, University of Birmingham, Birmingham, United Kingdom 12INFN Laboratori Nazionali di Frascati, Frascati, Italy 13Laboratoire de l'Accelerateur Lineaire (LAL), Orsay, France 14Instituto de Astrof´ısica de Canarias and Universidad de La Laguna, Departamento de Astrof´ısica, La Laguna, Tenerife, Spain 15CERN, CH-1211, Geneva 23, Switzerland 16Physik-Institut, Universit¨atZ¨urich,Z¨urich,Switzerland arXiv:1808.03477v2 [hep-ex] 31 Jul 2019 Abstract This report details the capabilities of LHCb and its upgrades towards the study of kaons and hyperons. -

Range of Usefulness of Bethe's Semiempirical Nuclear Mass Formula
RANGE OF ..USEFULNESS OF BETHE' S SEMIEMPIRIC~L NUCLEAR MASS FORMULA by SEKYU OBH A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment or the requirements tor the degree of MASTER OF SCIENCE June 1956 TTPBOTTDI Redacted for Privacy lrrt rtllrt ?rsfirror of finrrtor Ia Ohrr;r ef lrJer Redacted for Privacy Redacted for Privacy 0hrtrurn of tohoot Om0qat OEt ttm Redacted for Privacy Dru of 0rrdnrtr Sohdbl Drta thrrlr tr prrrEtrl %.ifh , 1,,r, ," r*(,-. ttpo{ by Brtty Drvlr ACKNOWLEDGMENT The author wishes to express his sincere appreciation to Dr. G. L. Trigg for his assistance and encouragement, without which this study would not have been concluded. The author also wishes to thank Dr. E. A. Yunker for making facilities available for these calculations• • TABLE OF CONTENTS Page INTRODUCTION 1 NUCLEAR BINDING ENERGIES AND 5 SEMIEMPIRICAL MASS FORMULA RESEARCH PROCEDURE 11 RESULTS 17 CONCLUSION 21 DATA 29 f BIBLIOGRAPHY 37 RANGE OF USEFULNESS OF BETHE'S SEMIEMPIRICAL NUCLEAR MASS FORMULA INTRODUCTION The complicated experimental results on atomic nuclei haYe been defying definite interpretation of the structure of atomic nuclei for a long tfme. Even though Yarious theoretical methods have been suggested, based upon the particular aspects of experimental results, it has been impossible to find a successful theory which suffices to explain the whole observed properties of atomic nuclei. In 1936, Bohr (J, P• 344) proposed the liquid drop model of atomic nuclei to explain the resonance capture process or nuclear reactions. The experimental evidences which support the liquid drop model are as follows: 1. Substantially constant density of nuclei with radius R - R Al/3 - 0 (1) where A is the mass number of the nucleus and R is the constant of proportionality 0 with the value of (1.5! 0.1) x 10-lJcm~ 2. -

Dark Matter in Nuclear Physics
Dark Matter in Nuclear Physics George Fuller (UCSD), Andrew Hime (LANL), Reyco Henning (UNC), Darin Kinion (LLNL), Spencer Klein (LBNL), Stefano Profumo (Caltech) Michael Ramsey-Musolf (Caltech/UW Madison), Robert Stokstad (LBNL) Introduction A transcendent accomplishment of nuclear physics and observational cosmology has been the definitive measurement of the baryon content of the universe. Big Bang Nucleosynthesis calculations, combined with measurements made with the largest new telescopes of the primordial deuterium abundance, have inferred the baryon density of the universe. This result has been confirmed by observations of the anisotropies in the Cosmic Microwave Background. The surprising upshot is that baryons account for only a small fraction of the observed mass and energy in the universe. It is now firmly established that most of the mass-energy in the Universe is comprised of non-luminous and unknown forms. One component of this is the so- called “dark energy”, believed to be responsible for the observed acceleration of the expansion rate of the universe. Another component appears to be composed of non-luminous material with non-relativistic kinematics at the current epoch. The relationship between the dark matter and the dark energy is unknown. Several national studies and task-force reports have found that the identification of the mysterious “dark matter” is one of the most important pursuits in modern science. It is now clear that an explanation for this phenomenon will require some sort of new physics, likely involving a new particle or particles, and in any case involving physics beyond the Standard Model. A large number of dark matter studies, from theory to direct dark matter particle detection, involve nuclear physics and nuclear physicists. -

Nuclear Physics
Massachusetts Institute of Technology 22.02 INTRODUCTION to APPLIED NUCLEAR PHYSICS Spring 2012 Prof. Paola Cappellaro Nuclear Science and Engineering Department [This page intentionally blank.] 2 Contents 1 Introduction to Nuclear Physics 5 1.1 Basic Concepts ..................................................... 5 1.1.1 Terminology .................................................. 5 1.1.2 Units, dimensions and physical constants .................................. 6 1.1.3 Nuclear Radius ................................................ 6 1.2 Binding energy and Semi-empirical mass formula .................................. 6 1.2.1 Binding energy ................................................. 6 1.2.2 Semi-empirical mass formula ......................................... 7 1.2.3 Line of Stability in the Chart of nuclides ................................... 9 1.3 Radioactive decay ................................................... 11 1.3.1 Alpha decay ................................................... 11 1.3.2 Beta decay ................................................... 13 1.3.3 Gamma decay ................................................. 15 1.3.4 Spontaneous fission ............................................... 15 1.3.5 Branching Ratios ................................................ 15 2 Introduction to Quantum Mechanics 17 2.1 Laws of Quantum Mechanics ............................................. 17 2.2 States, observables and eigenvalues ......................................... 18 2.2.1 Properties of eigenfunctions .........................................