Braun (10 Pages)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

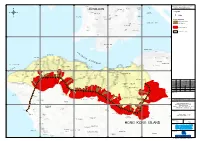
Drawing No MCL/P132/EIA/13-010
F¤w‹˛⁄s“„ SHA CHAU AND LUNG KWU CHAU MARINE PARK ¥ PAK CHAU 55 SHEUNG SHA CHAU F¨ SHA CHAU SHA CHAU17 AREA 6 p¤i SIU MO TO 6 øªÁ Cheung Sok Tsui AREA 4 30 øª 6 CHEUNG SOK U¤¤ Ha Kok Tsui j¤p¤ AREA 6 LUK KENG BAY j¤i THE BROTHERS TAI MO TO ‡ TAI6 MO TO Luk Keng YAM TSAI WAN YAN O WAN ±³ Yam Tsai ˝… 67 YAN O TSZ KAN CHAU ·¥ Ta Pang Po YªD YAN O TUK AREA 7 * Fª t Tung Yip Hang ¨¤w‹Œ Ser Res AsiaWorld-Expo ‘† AREA 3 Sham Shui Kok |¥ SZE PAK AU È«B¹ SKYCITY Ferry Terminal 263 p† »›·Œ LAI PIK SHAN NORTH CHEK LAP KOK “‚” T⁄ Golf Course SAM PAK AU Hong Kong International Airport j¤| È«B¹ TAI CHE TUNG Passenger Terminal o´ 302 Water Treatment Works ˆƒ⁄B Sewage Treatment –– Works T¤ Control Tower AREA 5 Sam Pak è¯Åª SIU HO WAN ”¤ Air Traffic 8 Control Complex ª¨P SAM PAK WAN NGAU TAU WAN G⁄ |© Chianti YI PAK AUfi CHEK LAP KOK Neo Horizon fiÆ… Siena hºá [ LAU FA TUNG DISCOVERY BAY YI PAK WAN (TAI PAK WAN) 378 ú©A »›·‚› Greenvale Hong Kong Aircraft Village Parkridge Village Engineering Ūl¶ Air Mail ˆX Centre WEST CHEK LAP KOK AREA 2 «½ Hai Kam Tsui r´º Headland Village 125 Discovery Bay 465 W¶Å¯@¤ ” j⁄ Super ƒŒ Terminal 1 * Tai Pak Tsui NORTH LANTAU HIGHWAY LO FU TAU `¯ Ȩw¬Åª TAI HO WAN Midvale VillageBeach AREA 7 Asia Airfreight Village ·‰ ˛¥Łfl Terminal Ferry Pier Business Aviation ˜ TSOI YUEN ˚› Centre 465 WAN Police ` Crestmont Villa 8 Post fi _Ä Peninsula Village La Costa Fª Parkvale Village U¿Æ [ƺ 117 ŪB¹ Fuel Tank ·‰ TUNG CHUNG ¥ Ferry Pier ” Airport Freight SCENIC HILL Pak Mong 299 Coastline Villa Forwarding Centre 77 fi M¬W TAI HO WAN d±z -
Hong Kong Island - 1 1
832000 834000 836000 838000 Central Park Copyright by Black & Veatch Hong Kong Limited Naval Base Hoi Fu Court Kowloon Map data reproduced with permission Lok Man TO KWA Rock Park Sun Chuen of the Director of Lands(C) Hong Kong Avenue KOWLOON HO MAN TIN WAN Chun Man Ho Man Tin Court Estate Legend Charming Garden To Kwa Wan YAU MA TEI Typhoon Shelter W1 King's Park Oi Man Hill Shafts New Yau Ma Tei Estate Sewage Treatment Works Typhoon Shelter Meteorological Kwun Tong Station Typhoon Shelter King's Park Villa Prosperous Garden KING'S PARK Tunnel Alignment Main Tunnel Alignments Ka Wai Hung Hom KOWLOON BAY Adits Alignments Chuen Estate Laguna Verde HUNG HOM Sorrento Intercepted Catchment Barracks Royal The Peninsula Whampoa Garden Waterfront 67 Subcatchment Boundary Victoria Tower 0 0 0 0 0 0 8 8 1 1 8 8 TSIM SHA TSUI TAI PAU MAI NORTH POINT North Point V Estate I C SAI YING PUN T O Healthy Village SAI WAN R Tanner Model I Garden Housing A Estate 42 H A R Pacific Palisades B O QUARRY BAY U R BRAEMAR HILL LITTLE GREEN ISLAND SHEK TONG TSUI Braemar Hill Mansions Causeway Bay SHEUNG WAN CENTRAL DISTRICT Typhoon Shelter L The Belcher's NE AN 5 CH 4 6 WAN CHAI 0 va 0 0 R W8 0 0 U 0 6 PH HKU1(P) 46 6 1 L 1 8 SU KENNEDY TOWN Sewage 8 Treatment RR1(P) Barracks Works CAUSEWAY BAY Sai Wan W10 Estate 3 MID-LEVELS vc Kung Man W11(P) 45 Tsuen Kwun Lung LUNG FU SHAN P5(P) 137 Lau 13 C 0 C 0 PFLR1(P) H Lai Tak 0 H 12 W5(P) A + TAI HANG A 0 Tsuen 7 Added Tunnel 8 + A W12(P) B 10/2005 LWG + C 5 H Scheme 0 H 0 00 0 0 240 A +0 C 8 0 VICTORIA P 7EAK + A EASTERN -

Appendix 9.2 Plant Species Recorded Within the Assessment Area
Appendix 9.2: Plant Species Recorded within the Assessment Area Agricultural Area Storm Water Fishponds Mudflat / Native/ Developed Distribution in Protection Village / Drain / Natural Modified and Coastal Scientific Name Growth Form Exotic to Area / Plantation Grassland Shrubland Woodland Marsh Mangrove Hong Kong (1) Status Orchard Recreational Watercourse Watercourse Mitigation Water Hong Kong Wasteland Dry Wet Pond Ponds Body Abrus precatorius climber: vine native common - + subshrubby Abutilon indicum native restricted - ++ herb Acacia auriculiformis tree exotic - - ++++ +++ + ++++ ++ +++ Acacia confusa tree exotic - - ++++ + +++ ++ ++ ++++ ++ ++++ Acanthus ilicifolius shrub native common - + ++++ Acronychia pedunculata tree native very common - ++ Adenosma glutinosum herb native very common - + + Adiantum capillus-veneris herb native common - + ++ ++ Adiantum flabellulatum herb native very common - + +++ +++ shrub or small Aegiceras corniculatum native common - +++ tree Aeschynomene indica shrubby herb native very common - + Ageratum conyzoides herb exotic common - ++ ++ ++ ++ ++ + Ageratum houstonianum herb exotic common - ++ + Aglaia odorata shrub exotic common - +++ + +++ + Aglaonema spp. herb - - - + + rare (listed under Forests and Ailanthus fordii (3) small tree native + Countryside Ordinance Cap. 96) Alangium chinense tree or shrub native common - ++ + ++ + +++ + Albizia lebbeck tree exotic - - +++ Alchornea trewioides shrub native common - + Aleurites moluccana tree exotic common - +++ ++ ++ ++ Allamanda cathartica climbing -

New Jan16.2011
Spring 2011 Mail Order Catalog Cistus Nursery 22711 NW Gillihan Road Sauvie Island, OR 97231 503.621.2233 phone 503.621.9657 fax order by phone 9 - 5 pst, visit 10am - 5pm, fax, mail, or email: [email protected] 24-7-365 www.cistus.com Spring 2011 Mail Order Catalog 2 USDA zone: 2 Symphoricarpos orbiculatus ‘Aureovariegatus’ coralberry Old fashioned deciduous coralberry with knock your socks off variegation - green leaves with creamy white edges. Pale white-tinted-pink, mid-summer flowers attract bees and butterflies and are followed by bird friendly, translucent, coral berries. To 6 ft or so in most any normal garden conditions - full sun to part shade with regular summer water. Frost hardy in USDA zone 2. $12 Caprifoliaceae USDA zone: 3 Athyrium filix-femina 'Frizelliae' Tatting fern An unique and striking fern with narrow fronds, only 1" wide and oddly bumpy along the sides as if beaded or ... tatted. Found originally in the Irish garden of Mrs. Frizell and loved for it quirkiness ever since. To only 1 ft tall x 2 ft wide and deciduous, coming back slowly in spring. Best in bright shade or shade where soil is rich. Requires summer water. Frost hardy to -40F, USDA zone 3 and said to be deer resistant. $14 Woodsiaceae USDA zone: 4 Aralia cordata 'Sun King' perennial spikenard The foliage is golden, often with red stems, and dazzling on this big and bold perennial, quickly to 3 ft tall and wide, first discovered in a department store in Japan by nurseryman Barry Yinger. Spikes of aralia type white flowers in summer are followed by purple-black berries. -

Supplement of Responses of Leaf Traits to Climatic Gradients: Adaptive Variation Vs
Supplement of Biogeosciences Discuss., 12, 7093–7124, 2015 http://www.biogeosciences-discuss.net/12/7093/2015/ doi:10.5194/bgd-12-7093-2015-supplement © Author(s) 2015. CC Attribution 3.0 License. Supplement of Responses of leaf traits to climatic gradients: adaptive variation vs. compositional shifts T.-T. Meng et al. Correspondence to: H. Wang ([email protected]) The copyright of individual parts of the supplement might differ from the CC-BY 3.0 licence. Supplement Figure S1: Partial residual plots for the relationships between leaf traits and the Cramer-Prentice moisture index (α), from a GLM analysis with PFT identity included as a predictor. Each point represents a species-site combination; fitted lines for each PFT are indicated by colours. Figure S2: Partial residual plots for the relationships between leaf traits and the Cramer-Prentice moisture index (α), from a GLM analysis with PFT × climate interactions included as predictors. Each point represents a species-site combination; fitted lines for each PFT are indicated by colours. Only significant PFT × climate interactions (P < 0.01) are shown. Figure S3: Partial residual plots for the relationships between leaf traits and growing degree days (GDD0), from a GLM analysis with PFT identity included as a predictor. Each point represents a species-site combination; fitted lines for each PFT are indicated by colours. Figure S4: Partial residual plots for the relationships between leaf traits and growing degree days (GDD0), from a GLM analysis with PFT × climate interactions included as predictors. Each point represents a species-site combination; fitted lines for each PFT are indicated by colours. -

C:\Mike's Documents\Book\Thirdedition
Insert as an Additional Taxa For Ardisia japonica: Ardisia crenata J. Sims Coralberry (Ardisia crenulata) C This is species is also known as Spiceberry; Coralberry is a larger version of A. japonica forming a rounded woody evergreen shrub 3N to 5N(6N) tall; the 4O to 6O(8O) long elliptic-lanceolate to oblanceolate lustrous dark green leaves have crenate-undulate margins; the foliage tends to be bunched toward the ends of the stems; the specific epithet refers to these crenate leaves. C Plants have small ¼O long white to pink inverted urn-shaped flowers in cymes in spring to summer; however, the species’ primary asset is the massed clusters of bright shiny red fruits; they are extremely showy, contrasting handsomely with the dark green foliage, ala a holly mimic. C Originating from Japan and Southeast Asia, this species has limited cold tolerance, but good heat tolerance’ it is effective in USDA zones 8(7) to 10 landscapes; plants are prolific seed producers and have naturalized extensively in eastern portions of the Gulf Coast; they are often found along woodland edges, but can tolerate full sun where adequate moisture is available; growth is best in well drained, moist, fertile, acidic to neutral soils; unfortunately, A. crenata can become weedy in favorable environments. C Ardisia crispa (C. Thunberg) A.P. de Candolle, Coral Ardisia, is a similar, but less cold hardy groundcover sometimes encountered along the Gulf Coast in South Texas or as an interiorscape plant. Copyrighted 2005 with all rights reserved by Michael A. Arnold; intended for future inclusion in Landscape Plants For Texas And Environs, Third Edition.. -

Rare Plants of Louisiana
Rare Plants of Louisiana Agalinis filicaulis - purple false-foxglove Figwort Family (Scrophulariaceae) Rarity Rank: S2/G3G4 Range: AL, FL, LA, MS Recognition: Photo by John Hays • Short annual, 10 to 50 cm tall, with stems finely wiry, spindly • Stems simple to few-branched • Leaves opposite, scale-like, about 1mm long, barely perceptible to the unaided eye • Flowers few in number, mostly born singly or in pairs from the highest node of a branchlet • Pedicels filiform, 5 to 10 mm long, subtending bracts minute • Calyx 2 mm long, lobes short-deltoid, with broad shallow sinuses between lobes • Corolla lavender-pink, without lines or spots within, 10 to 13 mm long, exterior glabrous • Capsule globe-like, nearly half exerted from calyx Flowering Time: September to November Light Requirement: Full sun to partial shade Wetland Indicator Status: FAC – similar likelihood of occurring in both wetlands and non-wetlands Habitat: Wet longleaf pine flatwoods savannahs and hillside seepage bogs. Threats: • Conversion of habitat to pine plantations (bedding, dense tree spacing, etc.) • Residential and commercial development • Fire exclusion, allowing invasion of habitat by woody species • Hydrologic alteration directly (e.g. ditching) and indirectly (fire suppression allowing higher tree density and more large-diameter trees) Beneficial Management Practices: • Thinning (during very dry periods), targeting off-site species such as loblolly and slash pines for removal • Prescribed burning, establishing a regime consisting of mostly growing season (May-June) burns Rare Plants of Louisiana LA River Basins: Pearl, Pontchartrain, Mermentau, Calcasieu, Sabine Side view of flower. Photo by John Hays References: Godfrey, R. K. and J. W. Wooten. -

Florida Exotic Pest Plant Councils 2017 List Of
CATEGORY II (continued) Gov. The 2017 list was prepared by the Scientific Name** Common Name List Zone FLEPPC List Definitions: Exotic – a species FLEPPC Plant List Committee Florida Exotic Pest Plant Tradescantia spathacea oyster plant C, S introduced to Florida, purposefully or accidentally, from a (Rhoeo spathacea, Rhoeo discolor) natural range outside of Florida. Native – a species Patricia L. Howell, Chair 2012-2017, Broward Tribulus cistoides puncture vine, burr-nut N, C, S Council’s 2017 List of whose natural range includes Florida. Naturalized County Parks, Natural Resources and Land Vitex trifolia simple-leaf chaste tree C, S Management Section, [email protected] Washingtonia robusta Washington fan palm C, S exotic – an exotic that sustains itself outside cultivation Invasive Plant Species Wisteria sinensis Chinese wisteria N, C (it is still exotic; it has not “become” native). Invasive Stephen H. Brown, UF / IFAS Lee County Xanthosoma sagittifolium malanga, elephant ear N, C, S exotic – an exotic that not only has naturalized, Extension, Parks and Recreation Division, The mission of the Florida Exotic Pest Plant but is expanding on its own in Florida native plant [email protected] Council is to support the management of invasive Recent changes to plant names exotic plants in Florida’s natural areas by communities. Janice Duquesnel, Florida Park Service, Florida providing a forum for the exchange of scientific, Department of Environmental Protection, educational and technical information. Old Name New Name Abbreviations: Government List (Gov. List): [email protected] www.fleppc.org Possession, propagation, sale, and/or transport of Aleurites fordii Vernicia fordii David W. -

Exempted Trees List
Prohibited Plants List The following plants should not be planted within the City of North Miami. They do not require a Tree Removal Permit to remove. City of North Miami, 2017 Comprehensive List of Exempted Species Pg. 1/4 Scientific Name Common Name Abrus precatorius Rosary pea Acacia auriculiformis Earleaf acacia Adenanthera pavonina Red beadtree, red sandalwood Aibezzia lebbek woman's tongue Albizia lebbeck Woman's tongue, lebbeck tree, siris tree Antigonon leptopus Coral vine, queen's jewels Araucaria heterophylla Norfolk Island pine Ardisia crenata Scratchthroat, coral ardisia Ardisia elliptica Shoebutton, shoebutton ardisia Bauhinia purpurea orchid tree; Butterfly Tree; Mountain Ebony Bauhinia variegate orchid tree; Mountain Ebony; Buddhist Bauhinia Bischofia javanica bishop wood Brassia actino-phylla schefflera Calophyllum antillanum =C inophyllum Casuarina equisetifolia Australian pine Casuarina spp. Australian pine, sheoak, beefwood Catharanthus roseus Madagascar periwinkle, Rose Periwinkle; Old Maid; Cape Periwinkle Cestrum diurnum Dayflowering jessamine, day blooming jasmine, day jessamine Cinnamomum camphora Camphortree, camphor tree Colubrina asiatica Asian nakedwood, leatherleaf, latherleaf Cupaniopsis anacardioides Carrotwood Dalbergia sissoo Indian rosewood, sissoo Dioscorea alata White yam, winged yam Pg. 2/4 Comprehensive List of Exempted Species Scientific Name Common Name Dioscorea bulbifera Air potato, bitter yam, potato vine Eichhornia crassipes Common water-hyacinth, water-hyacinth Epipremnum pinnatum pothos; Taro -

Spring 2011 Mail Order Catalog Cistus Nursery
Spring 2011 Mail Order Catalog Cistus Nursery 22711 NW Gillihan Road Sauvie Island, OR 97231 503.621.2233 phone 503.621.9657 fax order by phone 9 - 5 pst, visit 10am - 5pm, fax, mail, or email: [email protected] 24-7-365 www.cistus.com Spring 2011 Mail Order Catalog 2 USDA zone: 2 Symphoricarpos orbiculatus ‘Aureovariegatus’ coralberry $12 Caprifoliaceae USDA zone: 3 Athyrium filix-femina 'Frizelliae' tatting fern $14 Woodsiaceae USDA zone: 4 Aralia cordata 'Sun King' perennial spikenard $22 Araliaceae Aurinia saxatilis 'Dudley Nevill Variegated' $14 Brassicaceae Chrysanthemum x rubellum ‘Clara Curtis’ $11 Asteraceae Cyclamen hederifolium - silver shades $12 Primulaceae Eryngium bourgatii mediterranean sea holly $6 Apiaceae Euonymus europaeus ‘Red Ace’ spindle tree $14 Celastraceae Heuchera 'Sugar Plum' PPAF purple coral bells $12 Saxifragaceae Hydrangea macrophylla 'David Ramsey' big-leaf hydrangea $16 Hydrangeaceae Kerria japonica 'Albescens' white japanese kerria -$15 Rosaceae Liriope ‘Silver Dragon’ variegated lily turf $12 Liliaceae Opuntia basilaris ‘Peachy’ beavertail cactus $12 Cactaceae Opuntia fragilis SBH 6778 brittle prickly pear $7 Cactaceae Opuntia humifusa - dwarf from Claude Barr $12 Cactaceae Opuntia polyacantha 'Imnaha Sunset' $12 Cactaceae Opuntia polyacantha x ericacea var. columb. 'Golden Globe' $15 Cactaceae Opuntia x rutila - red/black spines $12 Cactaceae Philadelphus ‘Innocence’ mock orange $14 Hydrangeaceae Salix integra 'Hakuro-nishiki' dappled willow $12 Salicaceae Scilla scilloides chinese scilla $9 Liliaceae -

SDC Paper No. 5/2021 Annex 1
Annex 1 Hong Kong 12 February 2021 Mr. Lo Kin Hei Chairman Southern District Council Re: The Pok Fu Lam Conduit – Heritage status and improvement works Dear Chairman, We hope to raise an agenda item regarding the ‘Pok Fu Lam Conduit’ at the 8th SDC (2020- 2023) meeting scheduled at 2:30pm on 11 March 2021. Background The Pok Fu Lam Conduit was built between the Pokfulam Reservoir and the Albany Tanks during 1876 and 1877. It improved the supply of water to the City of Victoria. In 2004, based on Appraisal 429, Grade 2 Historic status was awarded to the No. 9 aqueduct of the Pok Fu Lam Conduit. Aspects of the conduit between the filter beds at west point (where the staff quarters and manager’s bungalow of the filter beds are now used as the Lung Fu Shan Environmental Education Centre) and the Albany tanks above Garden Road are hard to find. Part of Tank 2 is present at the corner of Caine, Bonham, Seymour and Hospital Road. The conduit was terminated at the filter beds in its early years and with construction of Conduit Road the alignment of that section disappeared from maps early last century. Between the Pok Fu Lam Reservoir and the former filter beds much of the conduit is present, and parts are still used for water supply. Of the 32 aqueducts bridging over ravines and gullies our survey found 16 to be present (8 in the Southern District, 8 in the Central and Western District): 1. Aqueducts No. 1 – 4 have been lost during construction of HKU properties (High West and Alberose). -

Information Note Strategic Cavern Area No. 40 – Pok Fu
- 1 - CAVERN MASTER PLAN – INFORMATION NOTE STRATEGIC CAVERN AREA NO. 40 – POK FU LAM This Information Note describes the characteristics, key development opportunities and constraints of Strategic Cavern Area No. 40 - Pok Fu Lam (the SCVA). It indicates the potential land uses suitable for cavern development within the area but would not pre-empt other possible land uses put forward by the project proponents with justifications. It also denotes the extent of potential portal locations. The spatial context of the SCVA is illustrated in the Reference Drawing appended to this Information Note. Reference should be made to the Explanatory Statement of the Cavern Master Plan for its background and purposes, as well as the definition and delineation criteria of SCVAs. 1. Location Plan Information Note (SCVA40 – Pok Fu Lam) - 2 - 2. Strategic Cavern Area Details Outline Zoning Plans (OZPs): Draft Pok Fu Lam OZP No. S/H10/16 Approved Mid-Levels West OZP No. S/H11/15 Draft The Peak Area OZP No. S/H14/12 Area: 86.1 ha Maximum elevation in the SCVA: +360 mPD Minimum elevation in the SCVA: +75 mPD 3. District Context Location The SCVA is located in the northwestern part of Hong Kong Island. It occupies the area of Lung Fu Shan in Mid-Levels. Sai Wan and Sai Ying Pun are to the north and northeast of the SCVA, Victoria Peak and Pok Fu Lam Country Park are to the east and south, Pok Fu Lam is to the southwest and Kennedy Town is to the west. The SCVA is generally hilly with a maximum elevation of about +360 mPD.