Bangor University DOCTOR of PHILOSOPHY Fragmented Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forest Conservation for Communities and Carbon: the Economics of Community Forest Management in The
Forest conservation for communities and carbon: the economics of community forest management in the Bale Mountains Eco-Region, Ethiopia Charlene Watson May 2013 Thesis submitted in fulfilment of the degree of Doctor of Philosophy London School of Economics and Political Science 1 Declaration of work This thesis is the result of my own work except where specifically indicated in the text and acknowledgements. The copyright of this thesis rests with the author. Quotation from it is permitted, provided that full acknowledgement is made. This thesis may not be reproduced without my prior written consent. Photos are the authors own, as are the figures generated. I warrant that this authorisation does not, to the best of my belief, infringe the rights of any third party. May 2013 2 Abstract Forest conservation based on payments anchored to opportunity costs (OCs) is receiving increasing attention, including for international financial transfers for reduced emissions from deforestation and degradation (REDD+). REDD+ emerged as a payment for environmental service (PES) approach in which conditional payments are made for demonstrable greenhouse gas emission reductions against a business-as-usual baseline. Quantitative assessments of the OCs incurred by forest users of these reductions are lacking. Existing studies are coarse, obscure the heterogeneity of OCs and do not consider how OCs may change over time. An integrated assessment of OCs and carbon benefits under a proposed community forest management (CFM) intervention linked to REDD+ is undertaken in Ethiopia. The OCs of land for the intervention are estimated through household survey and market valuation. Scenarios explore how OCs are likely to change over the intervention given qualitative conservation goals and available land-use change information. -

7. Willingness to Pay for Systematic Management of Community Forests for Conservation of Non-Timber Forest
CHAPTER 7 WILLINGNESS TO PAY FOR SYSTEMATIC MANAGEMENT OF COMMUNITY FORESTS FOR CONSERVATION OF NON-TIMBER FOREST PRODUCTS IN NIGERIA’S RAINFOREST REGION Implications for poverty alleviation NNAEMEKA A. CHUKWUONE# AND CHUKWUEMEKA E. OKORJI## # Centre for Entrepreneurship and Development Research and Department of Agricultural Economics, University of Nigeria Nsukka ## Department of Agricultural Economics, University of Nigeria Nsukka E-mail: [email protected] Abstract. Despite the importance of non-timber forest products (NTFP) in sustaining livelihood and poverty smoothening in rural communities, they are highly depleted and poorly conserved. Besides, conservation initiatives in Nigeria to date are rarely participatory. Even community forests, the main source of NTFP, are poorly conserved. Therefore, to enhance participatory conservation initiatives, this study determines the willingness of households in forest communities in the rainforest region of Nigeria to pay for systematic management of community forests using the contingent-valuation method. A multistage random-sampling technique was used in selecting 180 respondent households used for the study. The value-elicitation format used was discrete choice with open-ended follow-up questions. A Tobit model with sample selection was used in estimating the bid function. The findings show that some variables such as wealth category, occupation, number of years of schooling and number of females in a household positively and significantly influence willingness to pay. Gender (male-headed households), start price of the valuation, number of males in a household and distance from home to forests negatively and significantly influence willingness to pay. Incorporating these findings in initiatives to organize the local community in systematic management of community forests for NTFP conservation will enhance participation and hence poverty alleviation. -

Plant Species and Functional Diversity Along Altitudinal Gradients, Southwest Ethiopian Highlands
Plant Species and Functional Diversity along Altitudinal Gradients, Southwest Ethiopian Highlands Dissertation Zur Erlangung des akademischen Grades Dr. rer. nat. Vorgelegt der Fakultät für Biologie, Chemie und Geowissenschaften der Universität Bayreuth von Herrn Desalegn Wana Dalacho geb. am 08. 08. 1973, Äthiopien Bayreuth, den 27. October 2009 Die vorliegende Arbeit wurde in dem Zeitraum von April 2006 bis October 2009 an der Universität Bayreuth unter der Leitung von Professor Dr. Carl Beierkuhnlein erstellt. Vollständiger Abdruck der von der Fakultät für Biologie, Chemie und Geowissenschaften der Universität Bayreuth zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Prüfungsausschuss 1. Prof. Dr. Carl Beierkuhnlein (1. Gutachter) 2. Prof. Dr. Sigrid Liede-Schumann (2. Gutachter) 3. PD. Dr. Gregor Aas (Vorsitz) 4. Prof. Dr. Ludwig Zöller 5. Prof. Dr. Björn Reineking Datum der Einreichung der Dissertation: 27. 10. 2009 Datum des wissenschaftlichen Kolloquiums: 21. 12. 2009 Contents Summary 1 Zusammenfassung 3 Introduction 5 Drivers of Diversity Patterns 5 Deconstruction of Diversity Patterns 9 Threats of Biodiversity Loss in the Ttropics 10 Objectives, Research Questions and Hypotheses 12 Synopsis 15 Thesis Outline 15 Synthesis and Conclusions 17 References 21 Acknowledgments 27 List of Manuscripts and Specification of Own Contribution 30 Manuscript 1 Plant Species and Growth Form Richness along Altitudinal Gradients in the Southwest Ethiopian Highlands 32 Manuscript 2 The Relative Abundance of Plant Functional Types along Environmental Gradients in the Southwest Ethiopian highlands 54 Manuscript 3 Land Use/Land Cover Change in the Southwestern Ethiopian Highlands 84 Manuscript 4 Climate Warming and Tropical Plant Species – Consequences of a Potential Upslope Shift of Isotherms in Southern Ethiopia 102 List of Publications 135 Declaration/Erklärung 136 Summary Summary Understanding how biodiversity is organized across space and time has long been a central focus of ecologists and biogeographers. -

Taxonomic Significance of Foliar Epidermal Characters in the Caesalpinoideae
Vol. 8(10), pp. 462-472, October 2014 DOI: 10.5897/AJPS2014.1219 Article Number: 1B57E3E48465 ISSN 1996-0824 African Journal of Plant Science Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJPS Full Length Research Paper Taxonomic significance of foliar epidermal characters in the Caesalpinoideae Aworinde David Olaniran1* and Fawibe Oluwasegun Olamide2 1Department of Biological Sciences, Ondo State University of Science and Technology, Okitipupa, Ondo State, Nigeria. 2Department of Biological Sciences, Federal University of Agriculture Abeokuta, Ogun State, Nigeria. Received 31 July 2014; Accepted 21 October 2014 A detailed morphological study of the leaf epidermis of some species in the genera Bauhinia Linn., Caesalpinia Linn. Daniellia Hutch. & Dalz. and Senna Linn in Nigeria was undertaken in search of useful and stable taxonomic characters. The study reveals several interesting epidermal features some of which are novel in the genera. Leaf epidermal characters such as epidermal cell types, stomata types and the presence of trichomes were constant in some species and variable in others, making them to be of great significance in determining the relationships among and within species. Stomata were amphistomatic in all the species except in Senna alata, Senna siamea and Senna siberiana which are epistomatic. The species showed variability in their stomata length, width, density and index, which was reflected in their taxonomic delimitations. Key words: Taxonomy, Leaf epidermis, Bauhinia, Caesalpinia, Daniellia, Senna. INTRODUCTION Caesalpinoideae is a large sub-family of about 150 woodland types and on anthills 150 to 1800 m high; their genera with 2200 to 3000 species of flowering plants in seeds serve as food and their shoot as vegetables. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -
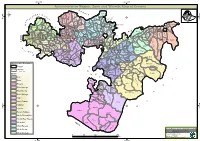
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: the State of the World's Forest Genetic Resources
ETHIOPIA This country report is prepared as a contribution to the FAO publication, The Report on the State of the World’s Forest Genetic Resources. The content and the structure are in accordance with the recommendations and guidelines given by FAO in the document Guidelines for Preparation of Country Reports for the State of the World’s Forest Genetic Resources (2010). These guidelines set out recommendations for the objective, scope and structure of the country reports. Countries were requested to consider the current state of knowledge of forest genetic diversity, including: Between and within species diversity List of priority species; their roles and values and importance List of threatened/endangered species Threats, opportunities and challenges for the conservation, use and development of forest genetic resources These reports were submitted to FAO as official government documents. The report is presented on www. fao.org/documents as supportive and contextual information to be used in conjunction with other documentation on world forest genetic resources. The content and the views expressed in this report are the responsibility of the entity submitting the report to FAO. FAO may not be held responsible for the use which may be made of the information contained in this report. THE STATE OF FOREST GENETIC RESOURCES OF ETHIOPIA INSTITUTE OF BIODIVERSITY CONSERVATION (IBC) COUNTRY REPORT SUBMITTED TO FAO ON THE STATE OF FOREST GENETIC RESOURCES OF ETHIOPIA AUGUST 2012 ADDIS ABABA IBC © Institute of Biodiversity Conservation (IBC) -

ACANTHACEAE 爵床科 Jue Chuang Ke Hu Jiaqi (胡嘉琪 Hu Chia-Chi)1, Deng Yunfei (邓云飞)2; John R
ACANTHACEAE 爵床科 jue chuang ke Hu Jiaqi (胡嘉琪 Hu Chia-chi)1, Deng Yunfei (邓云飞)2; John R. I. Wood3, Thomas F. Daniel4 Prostrate, erect, or rarely climbing herbs (annual or perennial), subshrubs, shrubs, or rarely small trees, usually with cystoliths (except in following Chinese genera: Acanthus, Blepharis, Nelsonia, Ophiorrhiziphyllon, Staurogyne, and Thunbergia), isophyllous (leaf pairs of equal size at each node) or anisophyllous (leaf pairs of unequal size at each node). Branches decussate, terete to angular in cross-section, nodes often swollen, sometimes spinose with spines derived from reduced leaves, bracts, and/or bracteoles. Stipules absent. Leaves opposite [rarely alternate or whorled]; leaf blade margin entire, sinuate, crenate, dentate, or rarely pinnatifid. Inflo- rescences terminal or axillary spikes, racemes, panicles, or dense clusters, rarely of solitary flowers; bracts 1 per flower or dichasial cluster, large and brightly colored or minute and green, sometimes becoming spinose; bracteoles present or rarely absent, usually 2 per flower. Flowers sessile or pedicellate, bisexual, zygomorphic to subactinomorphic. Calyx synsepalous (at least basally), usually 4- or 5-lobed, rarely (Thunbergia) reduced to an entire cupular ring or 10–20-lobed. Corolla sympetalous, sometimes resupinate 180º by twisting of corolla tube; tube cylindric or funnelform; limb subactinomorphic (i.e., subequally 5-lobed) or zygomorphic (either 2- lipped with upper lip subentire to 2-lobed and lower lip 3-lobed, or rarely 1-lipped with 3 lobes); lobes ascending or descending cochlear, quincuncial, contorted, or open in bud. Stamens epipetalous, included in or exserted from corolla tube, 2 or 4 and didyna- mous; filaments distinct, connate in pairs, or monadelphous basally via a sheath (Strobilanthes); anthers with 1 or 2 thecae; thecae parallel to perpendicular, equally inserted to superposed, spherical to linear, base muticous or spurred, usually longitudinally dehis- cent; staminodes 0–3, consisting of minute projections or sterile filaments. -

New Information on the Origins of Bottle Gourd (Lagenaria Siceraria)
New Information on the Origins of Bottle Gourd (Lagenaria siceraria) Item Type Article Authors Ellert, Mary Wilkins Publisher University of Arizona (Tucson, AZ) Journal Desert Plants Rights Copyright © Arizona Board of Regents. The University of Arizona. Download date 27/09/2021 04:31:57 Link to Item http://hdl.handle.net/10150/555919 8 Desert Plants of use by humans. Studies-both archeological and genetic, New Information on the Origins of seed and fruit-rind fragments indicate it had reached East of Bottle Gourd Asia 8,000 to 9,000 years before present (B.P.), that it was present as a domesticated plant in the New World by 10,000 (Lagenaria siceraria) B. P. and that it had a wide distribution in the Americas by 8,000 B.P. (Smith, 2000 and Erickson et. al, 2005) In the Southwestern US, bottle gourd most likely entered from Mary Wilkins Ellert Mexico as a domesticated plant at about the same time as 4433 W. Pyracantha Drive com (Zea mays) and squash (Cucurbita pepo)-by 3,500 to Tucson, Arizona 85741 4000 B.P.-and was widely grown as a container crop (Smith, [email protected] 2005). It is still grown today in the Sonoran Desert by the O'odam people, and seeds of the various traditional con tainer crop varieties are readily available through the Na " ........ always something new out of Africa" tive Seeds SEARCH group in Tucson, Arizona. History/Prehistory of Lagenaria In spite of its pan-tropical, pre-Columbian distribution, no Spanning continents, climates and cultures, the bottle gourd, evidence of the bottle gourd occurring in the wild on any Lagenaria siceraria (Mol.) Standley, Cucurbitaceae, has continent, as an indigenous part of the flora, rather than an served humans for thousands of years. -

Mt Mabu, Mozambique: Biodiversity and Conservation
Darwin Initiative Award 15/036: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems MT MABU, MOZAMBIQUE: BIODIVERSITY AND CONSERVATION November 2012 Jonathan Timberlake, Julian Bayliss, Françoise Dowsett-Lemaire, Colin Congdon, Bill Branch, Steve Collins, Michael Curran, Robert J. Dowsett, Lincoln Fishpool, Jorge Francisco, Tim Harris, Mirjam Kopp & Camila de Sousa ABRI african butterfly research in Forestry Research Institute of Malawi Biodiversity of Mt Mabu, Mozambique, page 2 Front cover: Main camp in lower forest area on Mt Mabu (JB). Frontispiece: View over Mabu forest to north (TT, top); Hermenegildo Matimele plant collecting (TT, middle L); view of Mt Mabu from abandoned tea estate (JT, middle R); butterflies (Lachnoptera ayresii) mating (JB, bottom L); Atheris mabuensis (JB, bottom R). Photo credits: JB – Julian Bayliss CS ‒ Camila de Sousa JT – Jonathan Timberlake TT – Tom Timberlake TH – Tim Harris Suggested citation: Timberlake, J.R., Bayliss, J., Dowsett-Lemaire, F., Congdon, C., Branch, W.R., Collins, S., Curran, M., Dowsett, R.J., Fishpool, L., Francisco, J., Harris, T., Kopp, M. & de Sousa, C. (2012). Mt Mabu, Mozambique: Biodiversity and Conservation. Report produced under the Darwin Initiative Award 15/036. Royal Botanic Gardens, Kew, London. 94 pp. Biodiversity of Mt Mabu, Mozambique, page 3 LIST OF CONTENTS List of Contents .......................................................................................................................... 3 List of Tables ............................................................................................................................. -

Cytotaxonomy of the Monopodial Orchids of the African and Malagasy Regions
Cytotaxonomy of the monopodial orchids of the African and Malagasy regions J. C. Arends & F. M. Van der Laan Department of Plant Taxonomy and Plant Geography, Agricultural University, Gen. Foulkesweg 37, 6700 ED Wageningen, The Netherlands Abstract The three subtribes which are recognized within the tribe Vandeae are represented in the tropical African and Malagasy regions. All taxa of the Vandeae have a monopodial growth habit. The first subtribe, Sarcanthinae, is mainly Asian-Australasian, but a few of its species occur in Madagascar and in Africa. The other two subtribes, Angraecinae and Aerangidinae, are both represented in the latter two regions. The Angraecinae ischaracterize d by the presence of a short rostellum, but this iselongate d in the Aerangidinae. According to earlier authors there is a correlation between presence of the short rostellum and a basic chromosome number of x= 19i nth e Angraecinae, and between the presence of an elongated rostellum and a basic number of x = 25 in the Aerangidinae. The results presented in this paper are placed in perspective with the chromosome numbers recorded by other authors. From the resulting chromosome number survey it appears that only part of the Angraecinae (Aeranthes, several species of Angraecum. Cryptopus and Jumellea) have a basic number of x = 19; some members of the Angraecinae (other species of Angraecum) were found to have a basic number of x = 21,24 and 25. The Aerangidinae is not characterized by a single basic number of x= 25 but by aserie s ranging from x = 23t o x= 27,o f which x= 23,24 and 25ar e the most frequent. -

Curriculum Vitae
Updated July 15, 2021 Curriculum Vitae GREGORY M. PLUNKETT ADDRESS: New York Botanical Garden Phone: (718) 817-8179 2900 Southern Blvd. FAX: (718) 817-8101 Bronx, NY 10458-5126 e-mail: [email protected] BIRTH DATE: February 21, 1965. Bayonne, New Jersey, USA EDUCATION: Ph.D. (Botany), Washington State University (D.E. Soltis, advisor). 1994. M.A. (Biology), The College of William and Mary in Virginia (G.W. Hall, advisor). 1990. B.S. (Biology), The College of William and Mary in Virginia. 1987. CURRENT POSITION: Director & Curator, Program for Molecular Systematics, New York Botanical Garden. PAST FACULTY POSITIONS Professor of Biology, Virginia Commonwealth University. 2008–2009. Associate Professor of Biology, Virginia Commonwealth University. 2002–2008. Assistant Professor of Biology, Virginia Commonwealth University. 1996–2002. CURRENT AFFILIATED POSITIONS: Affiliate Professor of Biology, Virginia Commonwealth University. 2009–present. Adjunct Professor of Plant Sciences, The Graduate Center, City University of New York. 2009–present. Adjunct Faculty, Fordham University. 2014–present. Research Associate, Missouri Botanical Garden, St. Louis. 2002–present. OTHER PROFESSIONAL EXPERIENCE: Curator, Herbarium of Virginia Commonwealth University (VCU). 1996–2009. Graduate Faculty, Molecular Biology and Genetics Program, VCU. 1998–2009. Fellow, Center for the Study of Biological Complexity, VCU. 2002–2009. Visiting Curator/Professeur, Muséum National d’Histoire Naturelle, Paris. 2004, 2005. Visiting Scientist, University of the South Pacific,