GATA2 Mutations Are Frequent in Intermediate-Risk Karyotype AML with Biallelic CEBPA Mutations and Are Associated with Favorable Prognosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functions of the Mineralocorticoid Receptor in the Hippocampus By
Functions of the Mineralocorticoid Receptor in the Hippocampus by Aaron M. Rozeboom A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Cellular and Molecular Biology) in The University of Michigan 2008 Doctoral Committee: Professor Audrey F. Seasholtz, Chair Professor Elizabeth A. Young Professor Ronald Jay Koenig Associate Professor Gary D. Hammer Assistant Professor Jorge A. Iniguez-Lluhi Acknowledgements There are more people than I can possibly name here that I need to thank who have helped me throughout the process of writing this thesis. The first and foremost person on this list is my mentor, Audrey Seasholtz. Between working in her laboratory as a research assistant and continuing my training as a graduate student, I spent 9 years in Audrey’s laboratory and it would be no exaggeration to say that almost everything I have learned regarding scientific research has come from her. Audrey’s boundless enthusiasm, great patience, and eager desire to teach students has made my time in her laboratory a richly rewarding experience. I cannot speak of Audrey’s laboratory without also including all the past and present members, many of whom were/are not just lab-mates but also good friends. I also need to thank all the members of my committee, an amazing group of people whose scientific prowess combined with their open-mindedness allowed me to explore a wide variety of interests while maintaining intense scientific rigor. Outside of Audrey’s laboratory, there have been many people in Ann Arbor without whom I would most assuredly have gone crazy. -

The Title of the Dissertation
UNIVERSITY OF CALIFORNIA SAN DIEGO Novel network-based integrated analyses of multi-omics data reveal new insights into CD8+ T cell differentiation and mouse embryogenesis A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Bioinformatics and Systems Biology by Kai Zhang Committee in charge: Professor Wei Wang, Chair Professor Pavel Arkadjevich Pevzner, Co-Chair Professor Vineet Bafna Professor Cornelis Murre Professor Bing Ren 2018 Copyright Kai Zhang, 2018 All rights reserved. The dissertation of Kai Zhang is approved, and it is accept- able in quality and form for publication on microfilm and electronically: Co-Chair Chair University of California San Diego 2018 iii EPIGRAPH The only true wisdom is in knowing you know nothing. —Socrates iv TABLE OF CONTENTS Signature Page ....................................... iii Epigraph ........................................... iv Table of Contents ...................................... v List of Figures ........................................ viii List of Tables ........................................ ix Acknowledgements ..................................... x Vita ............................................. xi Abstract of the Dissertation ................................. xii Chapter 1 General introduction ............................ 1 1.1 The applications of graph theory in bioinformatics ......... 1 1.2 Leveraging graphs to conduct integrated analyses .......... 4 1.3 References .............................. 6 Chapter 2 Systematic -

5045.Full.Pdf
IFN Consensus Sequence Binding Protein (Icsbp) Is Critical for Eosinophil Development This information is current as Maja Milanovic, Grzegorz Terszowski, Daniela Struck, of September 28, 2021. Oliver Liesenfeld and Dirk Carstanjen J Immunol 2008; 181:5045-5053; ; doi: 10.4049/jimmunol.181.7.5045 http://www.jimmunol.org/content/181/7/5045 Downloaded from References This article cites 47 articles, 33 of which you can access for free at: http://www.jimmunol.org/content/181/7/5045.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology IFN Consensus Sequence Binding Protein (Icsbp) Is Critical for Eosinophil Development1 Maja Milanovic,2* Grzegorz Terszowski,2† Daniela Struck,2‡ Oliver Liesenfeld,‡ and Dirk Carstanjen3* IFN consensus sequence binding protein (Icsbp) (IFN response factor-8) is a hematopoietic transcription factor with dual functions in myelopoiesis and immunity. -

GATA2 Regulates the Erythropoietin Receptor in T(12;21) ALL
GATA2 regulates the erythropoietin receptor in t(12;21) ALL Gaine, M. E., Sharpe, D. J., Smith, J. S., Colyer, H., Hodges, V. M., Lappin, T. R., & Mills, K. I. (2017). GATA2 regulates the erythropoietin receptor in t(12;21) ALL. Oncotarget, 8(39), 66061-66074. https://doi.org/10.18632/oncotarget.19792 Published in: Oncotarget Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights © 2017 The Authors. This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:04. Oct. 2021 www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 39), pp: 66061-66074 Research Paper GATA2 regulates the erythropoietin receptor in t(12;21) ALL Marie E. -

Identifying the Role of Wilms Tumor 1 Associated Protein in Cancer Prediction Using Integrative Genomic Analyses
MOLECULAR MEDICINE REPORTS 14: 2823-2831, 2016 Identifying the role of Wilms tumor 1 associated protein in cancer prediction using integrative genomic analyses LI‑SHENG WU1*, JIA-YI QIAN2*, MINGHAI WANG3* and HAIWEI YANG4 1Department of General Surgery, Anhui Provincial Hospital, Anhui Medical University, Hefei, Anhui 230001; 2Department of Breast Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029; 3Department of General Surgery, The First Affiliated Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241002; 4Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, P.R. China Received August 31, 2015; Accepted June 2, 2016 DOI: 10.3892/mmr.2016.5528 Abstract. The Wilms tumor suppressor, WT1 was first iden- regulatory factor 1, glucocorticoid receptor and peroxisome tified due to its essential role in the normal development of proliferator‑activated receptor γ transcription factor binding the human genitourinary system. Wilms tumor 1 associated sites were identified in the upstream (promoter) region of the protein (WTAP) was subsequently revealed to interact with human WTAP gene, suggesting that these transcription factors WT1 using yeast two‑hybrid screening. The present study may be involved in WTAP functions in tumor formation. identified 44 complete WTAP genes in the genomes of verte- brates, including fish, amphibians, birds and mammals. The Introduction vertebrate WTAP proteins clustered into the primate, rodent and teleost lineages using phylogenetic tree analysis. From The Wilms tumor suppressor gene WT1 was first identified 1,347 available SNPs in the human WTAP gene, 19 were due to its essential role in the normal development of the identified to cause missense mutations. -

The Novel Progesterone Receptor
0013-7227/99/$03.00/0 Vol. 140, No. 3 Endocrinology Printed in U.S.A. Copyright © 1999 by The Endocrine Society The Novel Progesterone Receptor Antagonists RTI 3021– 012 and RTI 3021–022 Exhibit Complex Glucocorticoid Receptor Antagonist Activities: Implications for the Development of Dissociated Antiprogestins* B. L. WAGNER†, G. POLLIO, P. GIANGRANDE‡, J. C. WEBSTER, M. BRESLIN, D. E. MAIS, C. E. COOK, W. V. VEDECKIS, J. A. CIDLOWSKI, AND D. P. MCDONNELL Department of Pharmacology and Cancer Biology (B.L.W., G.P., P.G., D.P.M.), Duke University Medical Center, Durham, North Carolina 27710; Molecular Endocrinology Group (J.C.W., J.A.C.), NIEHS, National Institutes of Health, Research Triangle Park, North Carolina 27709; Department of Biochemistry and Molecular Biology (M.B., W.V.V.), Louisiana State University Medical School, New Orleans, Louisiana 70112; Ligand Pharmaceuticals, Inc. (D.E.M.), San Diego, California 92121; Research Triangle Institute (C.E.C.), Chemistry and Life Sciences, Research Triangle Park, North Carolina 27709 ABSTRACT by agonists for DNA response elements within target gene promoters. We have identified two novel compounds (RTI 3021–012 and RTI Accordingly, we observed that RU486, RTI 3021–012, and RTI 3021– 3021–022) that demonstrate similar affinities for human progeste- 022, when assayed for PR antagonist activity, accomplished both of rone receptor (PR) and display equivalent antiprogestenic activity. As these steps. Thus, all three compounds are “active antagonists” of PR with most antiprogestins, such as RU486, RTI 3021–012, and RTI function. When assayed on GR, however, RU486 alone functioned as 3021–022 also bind to the glucocorticoid receptor (GR) with high an active antagonist. -

And EZH2-Regulated Gene, Has Differential Roles in AR-Dependent and -Independent Prostate Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 6, No.4 RUNX1, an androgen- and EZH2-regulated gene, has differential roles in AR-dependent and -independent prostate cancer Ken-ichi Takayama1,2, Takashi Suzuki3, Shuichi Tsutsumi4, Tetsuya Fujimura5, Tomohiko Urano1,2, Satoru Takahashi6, Yukio Homma5, Hiroyuki Aburatani4 and Satoshi Inoue1,2,7 1 Department of Anti-Aging Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan 2 Department of Geriatric Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan 3 Department of Pathology, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan 4 Genome Science Division, Research Center for Advanced Science and Technology (RCAST), The University of Tokyo, Meguro-ku, Tokyo, Japan 5 Department of Urology, Graduate School of Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan 6 Department of Urology, Nihon University School of Medicine, Itabashi-ku, Tokyo, Japan 7 Division of Gene Regulation and Signal Transduction, Research Center for Genomic Medicine, Saitama Medical University, Hidaka, Saitama, Japan Correspondence to: Satoshi Inoue, email: [email protected] Keywords: RUNX1, androgen receptor, EZH2, prostate cancer Received: October 02, 2014 Accepted: December 09, 2014 Published: December 10, 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Androgen receptor (AR) signaling is essential for the development of prostate cancer. Here, we report that runt-related transcription factor (RUNX1) could be a key molecule for the androgen-dependence of prostate cancer. We found RUNX1 is a target of AR and regulated positively by androgen. -

C/Ebpα Is an Essential Collaborator in Hoxa9/Meis1-Mediated Leukemogenesis
C/EBPα is an essential collaborator in Hoxa9/Meis1-mediated leukemogenesis Cailin Collinsa, Jingya Wanga, Hongzhi Miaoa, Joel Bronsteina, Humaira Nawera, Tao Xua, Maria Figueroaa, Andrew G. Munteana, and Jay L. Hessa,b,1 aDepartment of Pathology, University of Michigan, Ann Arbor, MI 48109; and bIndiana University School of Medicine, Indianapolis, IN 46202 Edited* by Louis M. Staudt, National Institutes of Health, Bethesda, MD, and approved May 19, 2014 (received for review February 12, 2014) Homeobox A9 (HOXA9) is a homeodomain-containing transcrip- with Hoxa9. In addition, C/EBP recognition motifs are enriched tion factor that plays a key role in hematopoietic stem cell expan- at Hoxa9 binding sites. sion and is commonly deregulated in human acute leukemias. A C/EBPα is a basic leucine-zipper transcription factor that plays variety of upstream genetic alterations in acute myeloid leukemia a critical role in lineage commitment during hematopoietic dif- −/− (AML) lead to overexpression of HOXA9, almost always in associ- ferentiation (18). Whereas Cebpa mice show complete loss of ation with overexpression of its cofactor meis homeobox 1 (MEIS1). the granulocytic compartment, recent work shows that loss of α A wide range of data suggests that HOXA9 and MEIS1 play a syn- C/EBP in adult HSCs leads to both an increase in the number ergistic causative role in AML, although the molecular mechanisms of functional HSCs and an increase in their proliferative and leading to transformation by HOXA9 and MEIS1 remain elusive. In repopulating capacity (19, 20). Conversely, CEBPA overexpression can promote transdifferentiation of a variety of fibroblastic cells to this study, we identify CCAAT/enhancer binding protein alpha (C/ the myeloid lineage and can induce monocytic differentiation in EBPα) as a critical collaborator required for Hoxa9/Meis1-mediated α MLL-fusion protein-mediated leukemias (21, 22). -

Letters to the Editor
LETTERS TO THE EDITOR The significant upregulation of Gata1 and EpoR mRNA Mice over-expressing human erythropoietin expression in hEPO over-expressing tg6 mice was con - indicate that erythropoietin enhances expression firmed by analyzing the spleen as a major source of of its receptor via up-regulated Gata1 and Tal1 hematopoiesis (Figure 2A and B). To further dissect the complexity of changes in the transcriptional network, the analysis of Myb mRNA expression served as marker for The development of medullary hematopoiesis is char - adult definitive erythroblasts, 10 showing significantly acterized by a specific expression profile of hematopoiet - ic transcription factors, including GATA transcription fac - tors. At mid-gestation, when hematopoiesis is newly A GATA 1 2 established in the bone marrow of human fetuses, initial - wt ly high GATA2 expression becomes subsequently down- regulated, while GATA1 expression increases in parallel. 1 1.5 Both transcription factors bind to overlapping sets of tg6 hematopoietic downstream target genes, often at distinct 1 sites, to regulate the balance between proliferation and differentiation. Chromatin occupancy by GATA1 and 0.5 GATA2 can change in the course of hematopoietic differ - entiation, leading to the so-called GATA switch. 2 Thus, a 0 n d7 d21 d49 i spatio-temporal regulation of GATA1 or GATA2 activities t c is required within lineage-specific differentiation. During a - erythroid differentiation GATA1 expression peaks at the b B GATA 2 3 o level of colony-forming units (CFU-E), where erythro - t 2 e poietin (Epo) exerts most specifically its effects, but v i t blocks terminal maturation if constitutively over- a 1.5 l 4 e expressed. -

Test Summary Flyer-NGS Panels.Pub
Cancer‐related Mutaon Analysis Next Generaon Gene Sequencing for Myeloid Tesng Assay Summary IU Health Molecular Pathology Laboratory now offers high throughput sequencing for hot spot mutations found in clinically relevant cancer genes. In addition to a general panel of 54 genes, selected panels have been developed for a more tai- lored application in specific cancers. Comparing to single gene assay, these panels offer a more comprehensive and eco- nomic way to assess prognosis and/or treatment options for cancer patients at the initial diagnosis or at the relapse. Orderable Name: Use IU Health Molecular Pathology requisition; Call 317.491.6417 for requisition. Panels include: AML Mutations by NGS ASXL1, CEBPA, DNMT3A, ETV6/TEL, FLT3, HRAS, IDH1, IDH2, KIT, KRAS, MLL, NPM1, NRAS, PHF6, RUNX1, TET2, TP53, WT1 MDS Mutations by NGS ASXL1, ATRX, BCOR, BCORL1, ETV6/TEL, DNMT3A, EZH2, GNAS, IDH1, IDH2, RUNX1, SF3B1, SRSF2, TET2, TP53, U2AF1, ZRSR2 CML Mutations by NGS ABL1 MPN Mutations by NGS ASXL1, BRAF, CALR, CSF3R, EZH2, IKZF1, JAK2, JAK3, KDM6A, KIT, MPL, PDGRA, SETBP1, TET2 CMML Mutations by NGS ASXL1, CBL, CBLB, CBLC, EZH2, RUNX1, TET2, TP53, SRSF2 JMML Mutations by NGS CBL, CBLB, CBLC, HRAS, KRAS, NRAS, PTPN11 ALL Mutations by NGS ABL1, CSF3R, FBXW7, IKZF1, JAK3, KDM6A, NOTCH1 CLL Mutations by NGS MYD88, NOTCH1, SF3B1, TP53 Lymphoma/Myeloma Mutations by NGS BRAF, CDKN2A, CSF3R, FBXW7, HRAS, KRAS, MYD88, NOTCH1, NRAS, SF3B1,TP53 Hematopoietic Neoplasms Mutations by NGS ABL1, ASXL1, ATRX, BCOR, BCORL1, BRAF, CALR, CBL, CBLB, CBLC,CDKN2A, CEBPA, CSF3R, CUX1, DNMT3A, ETV6/TEL, EZH2, FBXW7, FLT3, GATA1, GATA2, GNAS, HRAS, IDH1, IDH2, IKZF1,JAK2, JAK3, KDM6A, KIT, KRAS, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PDGFRA, PHF6, PTEN, PTPN11, RAD21, RUNX1,SETBP1, SF3B1, SMC1A, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, WT1, ZRSR2 Clinical Utility: This test is useful for the assessment of prognosis and/or treatment options for cancer patients at the initial diagnosis or at the relapse. -

Mutational Landscape and Clinical Outcome of Patients with De Novo Acute Myeloid Leukemia and Rearrangements Involving 11Q23/KMT2A
Mutational landscape and clinical outcome of patients with de novo acute myeloid leukemia and rearrangements involving 11q23/KMT2A Marius Billa,1,2, Krzysztof Mrózeka,1,2, Jessica Kohlschmidta,b, Ann-Kathrin Eisfelda,c, Christopher J. Walkera, Deedra Nicoleta,b, Dimitrios Papaioannoua, James S. Blachlya,c, Shelley Orwicka,c, Andrew J. Carrolld, Jonathan E. Kolitze, Bayard L. Powellf, Richard M. Stoneg, Albert de la Chapelleh,i,2, John C. Byrda,c, and Clara D. Bloomfielda,c aThe Ohio State University Comprehensive Cancer Center, Columbus, OH 43210; bAlliance for Clinical Trials in Oncology Statistics and Data Center, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210; cDivision of Hematology, Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210; dDepartment of Genetics, University of Alabama at Birmingham, Birmingham, AL 35294; eNorthwell Health Cancer Institute, Zucker School of Medicine at Hofstra/Northwell, Lake Success, NY 11042; fDepartment of Internal Medicine, Section on Hematology & Oncology, Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, NC 27157; gDepartment of Medical Oncology, Dana-Farber/Partners Cancer Care, Boston, MA 02215; hHuman Cancer Genetics Program, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210; and iDepartment of Cancer Biology and Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210 Contributed by Albert de la Chapelle, August 28, 2020 (sent for review July 17, 2020; reviewed by Anne Hagemeijer and Stefan Klaus Bohlander) Balanced rearrangements involving the KMT2A gene, located at patterns that include high expression of HOXA genes and thereby 11q23, are among the most frequent chromosome aberrations in contribute to leukemogenesis (14–16). -
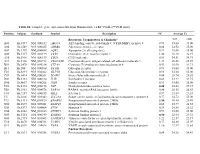
Table S1. Complete Gene Expression Data from Human Diabetes RT² Profiler™ PCR Array Receptors, Transporters & Channels* A
Table S1. Complete gene expression data from Human Diabetes RT² Profiler™ PCR Array Position Unigene GenBank Symbol Description FC Average Ct Receptors, Transporters & Channels* NGT GDM A01 Hs,5447 NM_000352 ABCC8 ATP-binding cassette, sub-family C (CFTR/MRP), member 8 0.93 35.00 35.00 A04 0Hs,2549 NM_000025 ADRB3 Adrenergic, beta-3-, receptor 0.88 34.92 35.00 A07 Hs,1307 NM_000486 AQP2 Aquaporin 2 (collecting duct) 0.93 35.00 35.00 A09 30Hs,5117 NM_001123 CCR2 Chemokine (C-C motif) receptor 2 1.00 26.28 26.17 A10 94Hs,5916 396NM_006139 CD28 CD28 molecule 0.81 34.51 34.71 A11 29Hs,5126 NM_001712 CEACAM1 Carcinoembryonic antigen-related cell adhesion molecule 1 1.31 26.08 25.59 B01 82Hs,2478 NM_005214 CTLA4 (biliaryCytotoxic glycoprotein) T-lymphocyte -associated protein 4 0.53 30.90 31.71 B11 24Hs,208 NM_000160 GCGR Glucagon receptor 0.93 35.00 35.00 C01 Hs,3891 NM_002062 GLP1R Glucagon-like peptide 1 receptor 0.93 35.00 35.00 C07 03Hs,6434 NM_000201 ICAM1 Intercellular adhesion molecule 1 0.84 28.74 28.89 D02 47Hs,5134 NM_000418 IL4R Interleukin 4 receptor 0.64 34.22 34.75 D06 57Hs,4657 NM_000208 INSR Insulin receptor 0.93 35.00 35.00 E05 44Hs,4312 NM_006178 NSF N-ethylmaleimide-sensitive factor 0.48 28.42 29.37 F08 79Hs,2961 NM_004578 RAB4A RAB4A, member RAS oncogene family 0.88 20.55 20.63 F10 69Hs,7287 NM_000655 SELL Selectin L 0.97 23.89 23.83 F11 56Hs,3806 NM_001042 SLC2A4 Solute carrier family 2 (facilitated glucose transporter), member 4 0.77 34.72 35.00 F12 91Hs,5111 NM_003825 SNAP23 Synaptosomal-associated protein, 23kDa 3.90