Altered Connection Properties of Important Network Hubs May Be
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface. -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -
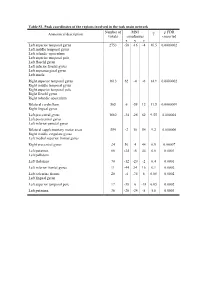
Table S1. Peak Coordinates of the Regions Involved in the Task Main
Table S1. Peak coordinates of the regions involved in the task main network Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Left superior temporal gyrus 2753 -58 -16 -4 16.5 0.0000002 Left middle temporal gyrus Left rolandic operculum Left superior temporal pole Left Heschl gyrus Left inferior frontal gyrus Left supramarginal gyrus Left insula Right superior temporal gyrus 1613 62 -4 -6 14.9 0.0000002 Right middle temporal gyrus Right superior temporal pole Right Heschl gyrus Right rolandic operculum Bilateral cerebellum 565 6 -58 12 11.5 0.0000009 Right lingual gyrus Left precentral gyrus 1682 -34 -26 62 9.55 0.000004 Left postcentral gyrus Left inferior parietal gyrus Bilateral supplementary motor areas 554 -2 10 54 9.2 0.000006 Right middle cingulate gyrus Left medial superior frontal gyrus Right precentral gyrus 24 56 4 44 6.9 0.00007 Left putamen 66 -24 -6 44 6.6 0.0001 Left pallidum Left thalamus 70 -12 -20 -2 6.4 0.0001 Left inferior frontal gyrus 11 -44 24 16 6.1 0.0002 Left calcarine fissure 20 -4 -74 8 6.08 0.0002 Left lingual gyrus Left superior temporal pole 17 -38 6 -18 6.05 0.0002 Left putamen 36 -28 -24 -8 5.8 0.0003 Table S2. Peak coordinates of the regions showing a main effect of item type Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Words – Pseudowords Left inferior frontal gyrus 96 -40 24 -6 7.6 0.000001 Left insula Left superior temporal pole Left middle temporal gyrus 141 -56 -36 -2 5.9 0.000015 Left precentral gyrus 26 -50 8 32 4.9 0.00025 Left inferior -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -
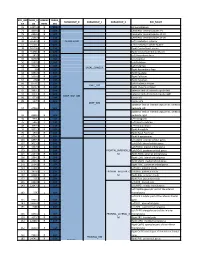
Core-Example1.Pdf
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Subtemporal Transparahippocampal Amygdalohippocampectomy for Surgical Treatment of Mesial Temporal Lobe Epilepsy Technical Note
Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy Technical note T. S. Park, M.D., Blaise F. D. Bourgeois, M.D., Daniel L. Silbergeld, M.D., and W. Edwin Dodson, M.D. Department of Neurology and Neurological Surgery, Washington University School of Medicine, and St. Louis Children's Hospital, St. Louis, Missouri Amygdalohippocampectomy (AH) is an accepted surgical option for treatment of medically refractory mesial temporal lobe epilepsy. Operative approaches to the amygdala and hippocampus that previously have been reported include: the sylvian fissure, the superior temporal sulcus, the middle temporal gyrus, and the fusiform gyrus. Regardless of the approach, AH permits not only extirpation of an epileptogenic focus in the amygdala and anterior hippocampus, but interruption of pathways of seizure spread via the entorhinal cortex and the parahippocampal gyrus. The authors report a modification of a surgical technique for AH via the parahippocampal gyrus, in which excision is limited to the anterior hippocampus, amygdala and parahippocampal gyrus while preserving the fusiform gyrus and the rest of the temporal lobe. Because transparahippocampal AH avoids injury to the fusiform gyrus and the lateral temporal lobe, it can be performed without intracarotid sodium amobarbital testing of language dominance and language mapping. Thus the operation would be particularly suitable for pediatric patients in whom intraoperative language mapping before resection is difficult. Key Words * amygdalohippocampectomy * complex partial seizure * parahippocampal gyrus * subtemporal approach Currently several different variations of temporal lobe resections are used for medically intractable complex partial seizures.[4,6,8,18,21,30,34] Among these operations is amygdalohippocampectomy (AH), first described in 1958 by Niemeyer,[16] who approached the amygdala and hippocampus through an incision on the middle temporal gyrus. -

BOLD Fmri Activation Induced by Vagus Nerve Stimulation in Seizure Patients W-C Liu, K Mosier, a J Kalnin, D Marks
811 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.6.811 on 1 June 2003. Downloaded from SHORT REPORT BOLD fMRI activation induced by vagus nerve stimulation in seizure patients W-C Liu, K Mosier, A J Kalnin, D Marks ............................................................................................................................. J Neurol Neurosurg Psychiatry 2003;74:811–813 to vagus nerve stimulation. Vagus nerve stimulation of Objective: To identify the cerebral activated regions asso- the thalamus may play an important role in controlling ciated with the vagus nerve stimulation in epilepsy seizures. patients. In addition to vagus nerve stimulation, studies using direct Design: Blood oxygenation level dependent functional electrical stimulation of the anterior4 or centromedian5 thala- magnetic resonance imaging (BOLD fMRI) was employed mus were reported to be potential treatments for refractory to detect areas of the brain activated by vagus nerve stimu- epilepsy. lation in five patients with documented complex partial sei- Recently, brain activation by vagus nerve stimulation was zures. detected using blood oxygenation level dependent functional Methods: Functional MRI was done on a GE 1.5T Echos- magnetic resonance imaging (BOLD fMRI).6 BOLD fMRI is a peed horizon scanner. Before each patient entered the non-invasive technique that uses local changes in the concen- scanner, the vagal nerve stimulator was set to a specific trations of oxyhaemoglobin and deoxyhaemoglobin to iden- ON–OFF paradigm so that the data could be analysed tify regions of increased neural activity.7 Our aim in the using a box-car type of design. The brains were scanned present study was to identify cerebral regions activated during both anatomically and functionally. -

Neural Processes in the Human Temporoparietal Cortex Separated by Localized Independent Component Analysis
9432 • The Journal of Neuroscience, June 24, 2015 • 35(25):9432–9445 Behavioral/Cognitive Neural Processes in the Human Temporoparietal Cortex Separated by Localized Independent Component Analysis X Kajsa M. Igelstro¨m, Taylor W. Webb, and Michael S.A. Graziano Princeton Neuroscience Institute and Department of Psychology, Princeton University, Princeton, New Jersey 08544 The human temporoparietal junction (TPJ) is a topic of intense research. Imaging studies have identified TPJ activation in association with many higher-order functions such as theory-of-mind, episodic memory, and attention, causing debate about the distribution of different processes. One major challenge is the lack of consensus about the anatomical location and extent of the TPJ. Here, we address this problem using data-driven analysis to test the hypothesis that the bilateral TPJ can be parcellated into subregions. We applied independent component analysis (ICA) to task-free fMRI data within a local region around the bilateral TPJ, iterating the ICA at multiple modelordersandinseveraldatasets.Thelocalizedanalysisallowedfinerseparationofprocessesandtheuseofmultipledimensionalities provided qualitative information about lateralization. We identified four subdivisions that were bilaterally symmetrical and one that was right biased. To test whether the independent components (ICs) reflected true subdivisions, we performed functional connectivity analysis using the IC coordinates as seeds. This confirmed that the subdivisions belonged to distinct networks. The right-biased -

Supplementary Materials
Supplementary Materials Supplementary Figure 1: TBSS results with covariates. FA on the white matter skeleton (green) was compared, with age and sex included as covariates in a general linear model. Significant results are indicated by the colour bar (p < 0.05, TFCE corrected). These are overlaid on the MNI standard template. Network Metric Case Control Uncorrected p Small-Worldness 0.8594 0.8506 0.2347 Average Node Strength 28.07 28.74 0.0769 Local Efficiency 0.3925 0.3993 0.1072 Global Efficiency 0.3885 0.3954 0.0927 Clustering Coefficient 0.3572 0.3652 0.0684 Modularity 0.0682 0.0655 0.3286 Characteristic Path Length 2.740 2.689 0.0829 Supplementary Table 1: Case-control comparison of network metrics. Network metrics were calculated from the FA-weighted structural network of each subJect. All network metrics were found to be normally distributed within each group (Kolmogorov-Smirnov test; p < 0.05). Columns show average network metrics for each group and the p-value calculated by a two-tailed, unpaired t-test. None of these differences were significant before correction so Bonferroni corrected p-values are not shown. Case Control Age Sex Design 1 0 6.75 0 0 1 7.25 1 … Contrast -1 1 0 0 Supplementary Table 2: Case-control comparison design matrix. The design matrix and contrast to test for reduced connectivity in cases compared to controls, with age and sex included as covariates, are shown. Case Control Metric (case) Metric (control) Age Sex Design 1 0 -7.5 0 6.75 0 0 1 0 5.47 7.25 1 … Contrasts 0 0 1 -1 0 0 0 0 -1 1 0 0 Supplementary Table 3: Correlation design matrix. -

Supplementary Information Unimodal Fmri Analyses and Results
Supplementary information Unimodal fMRI analyses and results Contrasts of interest in unimodal fMRI analyses included effects of Identity and Emotion. Increased activation for Partner > Stranger and Friend > Stranger was observed in right fusiform cortex, precuneus, posterior cingulate cortex, anterior cingulate cortex (ACC), right middle temporal gyrus and posterior superior temporal sulcus (Fig. S1). Additionally, the contrast Partner > Stranger showed activation in the bilateral orbitofrontal cortex, while Friend > Stranger yielded activation in the ventromedial prefrontal cortex. Emotion contrasts yielded activation for Happy > Neutral in the parieto-occipital cortex (including the intracalcarine cortex, lingual gyrus and precuneus), cerebellum and brain stem. Further clusters were located in the medial and ventromedial prefrontal cortex, orbitofrontal cortex, ACC, inferior and superior frontal gyrus, and insular cortex. Subcortically, activation was seen in the left amygdala, bilateral thalamus and left caudate. For Happy > Fear, activation was found in bilateral insula and frontal operculum, and left-lateralized inferior and middle frontal gyrus and lateral occipital cortex. See Table S1 for a complete list of activated brain regions. Fig. S1. Unimodal fMRI results. Bold activations for Identity comparisons (Partner > Stranger and Friend > Stranger) and Emotion comparisons (happy > neutral, happy > fear). Activation thresholded with FWE < 0.05 using permutations and threshold free cluster enhancement (TFCE). Table S1. Summary of