Study Protocol and Statistical Analysis Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Literature Review on Decentralization in Lesotho
Public Disclosure Authorized Kingdom of Lesotho Local Governance, Decentralization and Demand-Driven Service Delivery VOLUME II: ANNEXES Public Disclosure Authorized DRAFT REPORT - CONFIDENTIAL WORLD BANK Public Disclosure Authorized IN COLLABORATION WITH GOVERNMENT OF LESOTHO, GTZ, AND FAO JUNE 27, 2007 Public Disclosure Authorized Table of Contents ANNEX 1: LITERATURE REVIEW ON DECENTRALIZATION IN LESOTHO 3 ANNEX 2: DETAILED ASSESSMENT OF THE IMPLEMENTATION OF THE ACT 10 ANNEX 3A: STATUS OF IMPLEMENTATION OF THE PROVISIONS OF LOCAL GOVERNMENT ACT AS AMENDED .10 ANNEX 3.B STATUS OF IMPLEMENTATION OF THE ACT IN THE SECTORS ..........................................................18 ANNEX 3: CONCEPT PAPER ON CHANGE MANAGEMENT 27 ANNEX 4: PERCEPTIONS OF DECENTRALIZATION AT COMMUNITY AND DISTRICT LEVEL 31 ANNEX 4. 1 ADDITIONAL DETAILS ON METHODOLOGY, CCS AND VILLAGES ..................................................31 ANNEX 4.2 THE STORY OF MR POTSO CHALLENGING THE RIGHT TO FINE WITHOUT ISSUING RECEIPT ............32 ANNEX 5: PRIORITIES, ACCESS AND QUALITY OF SERVICES 33 ANNEX TABLE 5.1: PRIORITY AND ACCESS TO SERVICES ACROSS VILLAGES WITH DIFFERENT ROAD ACCESS ..33 ANNEX FIGURE 5.1: SERVICE PRIORITY IN THABA-TSEKA ...............................................................................34 ANNEX FIGURE 5.2: SERVICE ACCESS IN THABA-TSEKA..................................................................................35 ANNEX TABLE 5.2: STATUS OF SERVICES FOUND IN VILLAGES VISITED BY THE TEAM..................................36 ANNEX -

Lesotho Vulnerability Assessment Committee
2016 Lesotho Government Lesotho VAC Table of Contents List of Tables ................................LESOTHO................................................................ VULNERABILITY.............................................................................. 0 List of Maps ................................................................................................................................................................................ 0 Acknowledgments ................................ASSESSMENT................................................................ COMMITTEE................................................................ ... 3 Key Findings .............................................................................................................................................................................. 4 Executive Summary ................................................................................................................................................................ 5 INTERVENTION MODALITY SELECTION Section 1: Objectives, methodology and limitations ................................................................................................. 7 1.1 Objectives ................................In light ................................of the findings................................ from the LVAC................................ Market Assessment................................ that assessed....... 9 the functionality and performance of Lesotho’s food markets, LVAC proceeded to 1.2 Methodology -

Lesotho 2019 Human Rights Report
LESOTHO 2019 HUMAN RIGHTS REPORT EXECUTIVE SUMMARY Lesotho is a constitutional monarchy with a democratic parliamentary government. Under the constitution the king is head of state but does not actively participate in political activities. The prime minister is head of government and has executive authority. In 2017 former prime minister Pakalitha Mosisili of the Democratic Congress Party lost a vote of no confidence and a snap election. All major parties accepted the outcome, and Motsoahae Thomas Thabane of the All Basotho Convention Party (ABC) formed a coalition government and became prime minister. Mosisili transferred power peacefully to Thabane, and Mathibeli Mokhothu assumed leadership of the opposition. Local and international observers assessed the election as peaceful, credible, and transparent. The security forces consist of the Lesotho Defense Force (LDF), the Lesotho Mounted Police Service (LMPS), the National Security Service (NSS), and the Lesotho Correctional Service (LCS). The LMPS is responsible for internal security. The LDF maintains external security and may support police when the LMPS commissioner requests assistance. The NSS is an intelligence service that provides information on possible threats to internal and external security. The LDF and NSS report to the minister of defense; the LMPS, to the minister of police and public safety; and the LCS, to the minister of justice and correctional service. Civilian authorities generally maintained effective control over the security forces. The Southern African Development Community (SADC) Preventive Mission in Lesotho contingent of troops, deployed to foster stability as the government moved forward with SADC-recommended security-sector reforms, departed the country in November 2018. In May the government did not meet an SADC deadline for completion of constitutional and security reforms. -

Private Solutions for Infrastructure in Lesotho
A COUNTRY FRAMEWORK REPORT Private Solutions for Infrastructure in Lesotho PUBLIC-PRIVATE INFRASTRUCTURE ADVISORY FACILITY THE WORLD BANK A Country Framework Report Private Solutions for Infrastructure in Lesotho THE WORLD BANK Washington, D.C. Copyright © 2004 The findings, interpretations, and conclusions expressed in The International Bank for Reconstruction and Development/ this report are entirely those of the authors and should not be THE WORLD BANK attributed in any manner to the Public-Private Infrastructure 1818 H Street,NW Advisory Facility (PPIAF) or to the World Bank, to its affil- Washington, DC 20433, USA iated organizations, or to members of its Board of Executive Telephone 202-473-1000 Directors or the countries they represent. Internet www.worldbank.org Neither PPIAF nor the World Bank guarantees the accu- E-mail [email protected] racy of the data included in this publication or accepts All rights reserved responsibility for any consequence of their use. The bound- Manufactured in the aries, colors, denominations, and other information shown United States of America on any map in this report do not imply on the part of PPIAF or the World Bank Group any judgment on the legal status of any territory or the endorsement or acceptance of such boundaries. The material in this publication is copyrighted. Copyright is held by the World Bank on behalf of both the World Bank and PPIAF. Dissemination of this work is encouraged, and the World Bank will normally grant per- mission promptly and, when reproduction is for non-commercial purposes, without asking a fee. Permission to photocopy portions of this publication should be addressed to: Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, U.S.A., telephone 978-750-8400, fax 978-750-4470, or through the Internet at www.copyright.com For questions about this publication or information about ordering more copies, please refer to the PPIAF web- site or contact PPIAF c/o the World Bank, 1818 H. -

Mohale's Hoek District Council
MOHALE’S HOEK DISTRICT COUNCIL Information Handbook 2009 Handbook MOHALE’S HOEK DISTRICT COUNCIL LOCAL GOVERNMENT DISTRICT INFORMATION HANDBOOK June 2009 A COMPILATION OF CRUCIAL INFORMATION FOR THE DISTRICT (DISAGGREGATED COUNCIL-WISE) Ministry of local Government and Chieftainship German Technical Cooperation Department of Planning Kingdom of Lesotho Information Handbook 2009 1 handbook contents Prepared by: Department of Planning, Acronyms……………………………………………………………………………….............. 04 Ministry of Local Government and Chieftainship Mohale’s Hoek District Map………………………………………………………................. 05 Supported by: GTZ Lesotho Introduction………………………………………………………………………...........…….. 06 Background to Local Government in Lesotho……………………………............…………. 07 The Ministry of Local Government and Chieftainship Methodology…………………………………………………………………...........…………. 08 (MOLGC) through its Department of Planning, remains the owner of this handbook. However, there shall be no copy- 1. Profile of Community Councils……………………………………...……………… 09 right restrictions over the use or replication of the whole 2. Social Indicators……………………………………………………...……………… 10 or parts of this handbook. This is an approach for utiliza- 3. Gender Aspects……………………………………………………....………………. 11 tion by the interested parties and is entirely in the public domain. However, no changes shall be made 4. Agriculture………………………………………………………….………………… 12 to the handbook and reprinted thus without prior 5. Trade and Commerce…………………………………………………...…………… 13 permission of MOLGC. 6. Health…………………………………………………………………….…………… -

Decentralisation and Establishment of Local Government in Lesotho
Diplomarbeit Zur Prüfung im Studiengang Diplom Verwaltungswissenschaft An der Wirtschafts- und Sozialwissenschaftlichen Fakultät der Universität Potsdam Im Sommersemester 2006 Decentralisation and the Establishment of Local Government in Lesotho Vorgelegt am 09. Juli 2006 von _______________________________________________________________________ Robert Sperfeld Matrikel-Nr. 707036 Turnstraße 39, D-14482 Potsdam, 0331-88 74 37 8 [email protected] Betreut durch Dr. habil. Jochen Franzke Universität Potsdam Robert Sperfeld Decentralisation and Local Government in Lesotho ii Erklärung Hiermit versichere ich, dass ich die vorliegende Arbeit selbständig und ohne unerlaubte fremde Hilfe verfasst habe, und dass alle wörtlich oder sinngemäß aus Veröffentlichungen entnommenen Stellen dieser Arbeit unter Quellenangabe einzeln kenntlich gemacht sind. Potsdam, den 09. Juli 2006 Robert Sperfeld Robert Sperfeld Decentralisation and Local Government in Lesotho iii Table of Contents Erklärung..................................................................................................................ii Table of Contents....................................................................................................iii Text Boxes and Tables............................................................................................ v List of Appendixes ................................................................................................... v List of Abbreviations ................................................................................................vi -

Mcc Lesotho Compact 2008-2013 Evaluation Design
MCC LESOTHO COMPACT 2008-2013 MCA HEALTH PROJECT LESOTHO FINAL EVALUATION MCC-15-PO-0074 EVALUATION DESIGN June 16, 2017 HEALTHMATCH consultancies Pim de Graaf The Netherlands Page 1 of 96 Content CONTENT .......................................................................................................................................................... 2 ABBREVIATIONS AND ACRONYMS ................................................................................................................... 4 I INTRODUCTION AND BACKGROUND ........................................................................................................ 5 COUNTRY CONTEXT .................................................................................................................................................. 5 HEALTH PROFILE ...................................................................................................................................................... 6 HEALTH SYSTEM ...................................................................................................................................................... 7 OBJECTIVES OF THIS REPORT .................................................................................................................................... 12 2 OVERVIEW OF THE COMPACT ................................................................................................................ 13 THE HEALTH PROJECT AND IMPLEMENTATION PLAN ...................................................................................................... -

Highlights Contents
LESOTHO METEOROLOGICAL SERVICES (LEKALA LA TSA BOLEPI) Ten-Day Agrometeorological Bulletin 21st – 31st January 2005 Issue No.10/2004-05 Date of Issue: 4 February 2005 Vol. 3 …dedicated to the agricultural community … aimed at harmonizing agricultural activities with weather and climate Contents Highlights Weather Summary Page 1 q Below normal rains recorded. Rainfall Situation q Cumulative rainfall normal countrywide. Page 1 q Temperature Crops conditions good at few localities. Page 1 q Infestation of insects at some places. Crop Stage and Condition Page 1 q Low rainfall expected for next dekad. Dekadal Outlook Page 1 Rainfall and Temperature Summaries Page 2 Glossary Page 3 The Director TEL: (+266) 22324374/22324425 Lesotho Meteorological Services FAX: (+266) 22325057/22350325 Agrometeorological Section E-mail:[email protected] P.O. Box 14515 http://www.lesmet.org.ls Maseru 100, Lesotho Issue No. 10/2004-05 Vol.3 21st –31st January 2005 WEATHER SUMMARY The percentage departure from normal cumulative 11th – 20 th January 2005 rainfall ranges from -12% to 32% (Table 1). The highest cumulative rainfalls of 721.5mm, The last dekad of January was dominated by 543.9mm and 509.4mm are recorded at Oxbow, surface trough. However, there was insufficient Leribe and Qacha’s Nek (Table 1 and Fig. 3). moisture over the interior as a result only partly Mafeteng, Maseru Airport, Moshoeshoe I and cloudy and warm conditions with few Phuthiatsana stations are the only stations which thundershowers occurred. have received cumulative rainfall of less than 400mm. RAINFALL SITUATION TEMPERATURE The last ten days of January received relatively low rainfall compared to the previous dekad (11th th The country experienced near normal – 20 January 2005) which was very wet. -
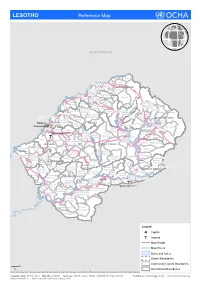
LESOTHO Reference Map
LESOTHO Reference Map SOUTH AFRICA Makhunoane Liqobong Likila Ntelle n Maisa-Phoka Ts'a-le- o d Moleka le BUTHA BUTHE a Lipelaneng C Nqechane/ Moteng Sephokong Linakeng Maputsoe Leribe Menkhoaneng Sekhobe Litjotjela Likhotola/ LERIBE Hleoheng Malaoaneng Manka/ Likhakeng Matlameng Mapholaneng/ Fobane Koeneng/ Phuthiatsana Kolojane Lipetu/ Kao Pae-la-itlhatsoa -Leribe Fenyane Litsilo -Pae-la-itlhatsoa Mokhachane/ Mamathe/Bulara Mphorosane Molika-liko Makhoroana Limamarela Tlhakanyu/Motsitseng Teyateyaneng Seshote Mapholaneng/ Majoe-Matso/ Meno/ Lekokoaneng/Maqhaka Mohatlane/ Sebetia/Khokhoba Pae-la-itlhatsoa Matsoku -Mapholaneng MOKHOTLONG Lipohong Thuapa-Kubu/ Moremoholo/ Katse Popa Senekane BEREA Moshemong Maseru Thuathe Koali/ Taung/Khubelu Mejametalana p Mokhameleli Semenanyane Mokhotlong S Maluba-lube/ Mateanong e Maseru Suoane/ m Rafolatsane Thaba-Bosiu g Ratau e Liphakoeng n Bokong n e a Ihlo-Letso/ Mazenod Maseru Moshoeshoe l Setibing/ Khotso-Ntso Sehong-hong e Tsoelike/ Moeketsane h Pontseng/ Makopoi/ k Mantsonyane Bobete p Popa_MSU a Likalaneng Mahlong Linakaneng M Thaba-Tseka/ Rothe Mofoka Nyakosoba/Makhaleng Maboloka Linakeng/Bokhoasa/Manamaneng THABA TSEKA Kolo/ MASERU Setleketseng/ Tebang/ Matsieng Tsakholo/Mapotu Seroeneg S Mashai e Boleka n Tsa-Kholo Ramabanta/ q Methalaneng/ Tajane Moeaneng u Rapo-le-boea n Khutlo-se-metsi Litsoeneng/Qalabane Maboloka/ y Sehonghong Thaba-Tsoeu/ Monyake a Lesobeng/ Mohlanapeng Mathebe/ n Sehlaba-thebe/ Thabaneng Ribaneng e Takalatsa Likhoele Moshebi/ Kokome/ MAFETENG Semonkong Leseling/ -

Competition Issues in the Transport Sector in Lesotho
UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Competition issues in the Transport Sector in Lesotho By Chabeli J. RAMOLISE New York and Geneva, 2011 The views expressed in this study are those of the author do not necessarily reflect the views of the UNCTAD Secretariat. NOTE UNCTAD serves as the focal point within the United Nations Secretariat for all matters related to competition policy. UNCTAD seeks to further the understanding of the nature of competition law and policy and its contribution to development and to create an enabling environment for an efficient functioning of markets. UNCTAD’s work is carried out through intergovernmental deliberations, capacity building activities, policy advice, and research and analysis on the interface between competition policy and development. UNCTAD’s work on competition law and policies falls within the framework of the Set of Multilaterally Agreed Principles and Rules for the Control of Restrictive Business Practices (the “United Nations Set of Principles and Rules on Competition”), adopted by the General Assembly in 1980. The set seeks, inter alia, to assist developing countries in adopting and enforcing effective competition law and policy that are suited to their development needs and economic situation. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Material in this publication may be freely quoted or reprinted, but acknowledgement is requested, together with a reference to the document number. -

Second State Of
Second State of the Environment 2002 Report Lesotho Lesotho Second State of the Environment Report 2002 Authors: Chaba Mokuku, Tsepo Lepono, Motlatsi Mokhothu Thabo Khasipe and Tsepo Mokuku Reviewer: Motebang Emmanuel Pomela Published by National Environment Secretariat Ministry of Tourism, Environment & Culture Government of Lesotho P.O. Box 10993, Maseru 100, Lesotho ISBN 99911-632-6-0 This document should be cited as Lesotho Second State of the Environment Report for 2002. Copyright © 2004 National Environment Secretariat. All rights reserved. No parts of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without prior permission of the publisher. Design and production by Pheko Mathibeli, graphic designer, media practitioner & chartered public relations practitioner Set in Century Gothic, Premium True Type and Optima Lesotho, 2002 3 Contents List of Tables 8 Industrial Structure: Sectoral Composition 34 List of Figures 9 Industrial Structure: Growth Rates 36 List of Plates 10 Population Growth 37 Acknowledgements 11 Rural to Urban Migration 37 Foreword 12 Incidence of Poverty 38 Executive Summary 14 Inappropriate Technologies 38 State and impacts: trends 38 Introduction 24 Human Development Trends 38 Poverty and Income Distribution 44 Socio-Economic and Cultural Environment. 26 Agriculture and Food Security 45 People, Economy and Development Ensuring Long and Healthy Lives 46 Socio-Economic Dimension 26 Ensuring -

Kingdom of Lesotho 2000 End Decade Multiple Indicator Cluster Survey (Emics)
KINGDOM OF LESOTHO 2000 END DECADE MULTIPLE INDICATOR CLUSTER SURVEY (EMICS) DRAFT PRELIMINARY REPORT Tuesday, May 28, 2002 Government of Lesotho Bureau of Statistics Post Office Box 455 MASERU End Decade Multiple Indicator Cluster Survey – Draft Preliminary Report Executive Summary This Draft Preliminary Report presents the initial results of the 2000 Lesotho End-Decade Multiple Indicator Cluster Survey (EMICS). These results were derived from a nationally representative survey of households, women, and children. The main objectives of the survey were: ð to provide up-to-date information for assessing the situation of children and women in Lesotho at the end of the decade, and; ð to furnish data needed for monitoring progress toward goals established at the World Summit for Children and as a basis for future action. It is organised in four main sections. Chapter One is a documentation of the background to the 2000 Lesotho EMICS, and its objectives. The technical details of the survey, including sampling procedures, data collection and analysis are reported in Chapter Two. Chapter Three presents an evaluation of the quality of the data collected during the survey and used to prepare the analysis presented in Chapter Four. The Appendices attach the questionnaire used in the survey, some key documentation and lists key personnel and organisations involved in the 2000 Lesotho EMICS. It is expected that this Draft Preliminary Report will generate discussion on the findings in respect of the health, education, and child labour situation in Lesotho amongst government agencies, non-governmental organisations (NGOs), multilateral donors, the press and the public. Primary School Attendance · Approximately sixty five percent of children of primary school age in Lesotho are attending primary school.