Antibody Cross-Reactivity in Antivenom Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Brazilian Scorpion Venoms on the Central Nervous System
Nencioni et al. Journal of Venomous Animals and Toxins including Tropical Diseases (2018) 24:3 DOI 10.1186/s40409-018-0139-x REVIEW Open Access Effects of Brazilian scorpion venoms on the central nervous system Ana Leonor Abrahão Nencioni1* , Emidio Beraldo Neto1,2, Lucas Alves de Freitas1,2 and Valquiria Abrão Coronado Dorce1 Abstract In Brazil, the scorpion species responsible for most severe incidents belong to the Tityus genus and, among this group, T. serrulatus, T. bahiensis, T. stigmurus and T. obscurus are the most dangerous ones. Other species such as T. metuendus, T. silvestres, T. brazilae, T. confluens, T. costatus, T. fasciolatus and T. neglectus are also found in the country, but the incidence and severity of accidents caused by them are lower. The main effects caused by scorpion venoms – such as myocardial damage, cardiac arrhythmias, pulmonary edema and shock – are mainly due to the release of mediators from the autonomic nervous system. On the other hand, some evidence show the participation of the central nervous system and inflammatory response in the process. The participation of the central nervous system in envenoming has always been questioned. Some authors claim that the central effects would be a consequence of peripheral stimulation and would be the result, not the cause, of the envenoming process. Because, they say, at least in adult individuals, the venom would be unable to cross the blood-brain barrier. In contrast, there is some evidence showing the direct participation of the central nervous system in the envenoming process. This review summarizes the major findings on the effects of Brazilian scorpion venoms on the central nervous system, both clinically and experimentally. -

Ecologia Do Tityus Serrulatus E Ação De Sua Peçonha No Corpo Humano
Centro Universitário de Brasília Faculdade de Ciências da Saúde ECOLOGIA DO TITYUS SERRULATUS E AÇÃO DE SUA PEÇONHA NO CORPO HUMANO Leonardo Carneiro de Morais Sá Brasília – 2002 Centro Universitário de Brasília Faculdade de Ciências da Saúde Licenciatura em Ciências Biológicas ECOLOGIA DO TITYUS SERRULATUS E AÇÃO DE SUA PEÇONHA NO CORPO HUMANO Leonardo Carneiro de Morais Sá Monografia apresentada à Faculdade de Ciências da Saúde do Centro Universitário de Brasília como parte dos requisitos para a obtenção do grau de Licenciado em Ciências Biológicas Orientador: Profª. Dra. Elizabeth Maria Mamede da Costa Brasília – 2002 2 DEDICATÓRIA A meus pais a quem devo tudo o que conquistei durante essa caminhada da vida Aos meu irmão Anderson e a minha irmã Fernanda que sempre me ajudaram, A Graziela minha companheira pelo apoio e admiração, E a Deus que me deu o Dom da vida e muita saúde para lutar e chegar ao fim desta batalha. 3 AGRADECIMENTOS Aos professores que me proporcionaram obter o conhecimento que hoje detenho. A Professora Simone Rios onde quer que esteja pelo apoio, pelo tempo gasto em função de minha obra e pelo incentivo. A Professora Elizabeth Maria Mamede da Costa pelas horas que esteve ao meu lado incentivando e orientando. A todos os amigos e colegas que compartilharam comigo as ansiedades e felicidades dessa caminhada. 4 RESUMO O Tityus Serrulatus é um escorpião pertencente a família Buthidae, que por possuir uma peçonha de alta toxidade se apresenta como uma das mais perigosas do mundo. É um animal tipicamente urbano, buscando abrigo dentro de residências, tubulações entre outros. -

Diagnostic Potential of the Haemagglutination
Bulgarian Journal of Veterinary Medicine (2007), 10 , N o 3, 169 −178 DIAGNOSTIC POTENTIAL OF THE HAEMAGGLUTINATION INHIBITION TEST, THE IMMUNODIFFUSION TEST AND ELISA FOR DETECTION OF ANTIBODIES IN CHICKENS, INTRAVENOUSLY INFECTED WITH A/DUCK/BULGARIA/05 H6N2 AVIAN INFLUENZA VIRUS ISOLATE I. S. ZARKOV Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria Summary Zarkov, I., 2007. Diagnostic potential of the haemagglutination inhibition test, the immuno- diffusion test and ELISA for detection of antibodies in chickens, intravenously infected with A/duck/Bulgaria/05 H6N2 avian influenza virus isolate. Bulg. J. Vet. Med. , 10 , No 3, 169 −178. After experimental infection of chickens with an avian influenza viral isolate A/duck/Bulgaria/05 H6N2, the potential of the haemagglutination inhibition test (HIT), the immunodiffusion test (IDT) and enzyme-linked immunosorbent assay (ELISA) for antibody detection was evaluated. The results evidenced that in birds, haemagglutinins, precipitins and IgG antibodies, detectable with ELISA, were formed. The percentage of chickens with subtype-specific antibodies was the highest by the 21 st day of infection (100 %), followed by the 14 th (66.7 %) and the 28 th (55.6 %) days, and was the lowest by the 7 th day (44.4 %). Serum titres ranged between 1:4 and 1:256 with predomination of 1:8 and 1:16 titres (29.2 % each). The mean arithmetic titre for the experiment was 1:38.2. The highest percentage of chickens with precipitins was observed by the 14th day (55.6 %) followed by the 7 th , 21 st and the 28 th days with 33.3 % each. -

Confirmation of Parthenogenesis in the Medically Significant, Synanthropic Scorpion Tityus Stigmurus (Thorell, 1876) (Scorpiones: Buthidae)
NOTA BREVE: Confirmation of parthenogenesis in the medically significant, synanthropic scorpion Tityus stigmurus (Thorell, 1876) (Scorpiones: Buthidae) Lucian K. Ross NOTA BREVE: Abstract: Parthenogenesis (asexuality) or reproduction of viable offspring without fertiliza- Confirmation of parthenogenesis tion by a male gamete is confirmed for the medically significant, synanthropic in the medically significant, synan- scorpion Tityus (Tityus) stigmurus (Thorell, 1876) (Buthidae), based on the litters thropic scorpion Tityus stigmurus of four virgin females (62.3–64.6 mm) reared in isolation in the laboratory since (Thorell, 1876) (Scorpiones: Buthi- birth. Mature females were capable of producing initial litters of 10–21 thely- dae) tokous offspring each; 93–117 days post-maturation. While Tityus stigmurus has been historically considered a parthenogenetic species in the pertinent literature, Lucian K. Ross the present contribution is the first to demonstrate and confirm thelytokous 6303 Tarnow parthenogenesis in this species. Detroit, Michigan 48210-1558 U.S.A. Keywords: Buthidae, Tityus stigmurus, parthenogenesis, reproduction, thelytoky. Phone/Fax: +1 (313) 285-9336 Mobile Phone: +1 (313) 898-1615 E-mail: [email protected] Confirmación de la partenogénesis en el escorpión sinantrópico y de relevan- cia médica Tityus stigmurus (Thorell, 1876) (Scorpiones: Buthidae) Resumen: Se confirma la partenogénesis (asexualidad) o reproducción con progenie viable Revista Ibérica de Aracnología sin fertilización mediante gametos masculinos en el escorpión sinantrópico y de ISSN: 1576 - 9518. relevancia médica Tityus stigmurus (Thorell, 1876) (Scorpiones: Buthidae). Cua- Dep. Legal: Z-2656-2000. tro hembras vírgenes (Mn 62.3-64.6) se criaron en el laboratorio desde su naci- Vol. 18 miento. Al alcanzar el estadio adulto tuvieron una descendencia telitoca inicial de Sección: Artículos y Notas. -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -
Notes on the Postembryonic Development of Tityus Melanostictus Pocock, 1893 (Scorpiones, Buthidae) from Trinidad 7- 13 ©Zoologisches Museum Hamburg
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg Jahr/Year: 2011 Band/Volume: 15 Autor(en)/Author(s): Lourenco Wilson R., Ythier Eric, Cloudsley- Thompson John L. Artikel/Article: Notes on the postembryonic development of Tityus melanostictus Pocock, 1893 (Scorpiones, Buthidae) from Trinidad 7- 13 ©Zoologisches Museum Hamburg, www.zobodat.at Entomol. Mitt. zool. Mus. Hamburg15(178): 7-13 Hamburg, 1. Juli 2008 ISSN 0044-5223 Notes on the postembryonic development of Tityus melanostictus Pocock, 1893 (Scorpiones, Buthidae) from Trinidad W ilson R. Lourenço, E ric Y thier & J ohn L. Cloudsley-Thompson (with 4 figures) Abstract Biological observations were made on Tityus melanostictus Pocock, 1893, based on specimens from the region of Cumana, Trinidad. The total duration of embryonic development can only be estimated as 2.5 to 3 months, while the molts necessary for the acquisition of the different juvenile instars and adulthood took place at ages that averaged: 4, 48, 119, 211, 265 and 330 days. These developmental periods are not greatly different from those in other average sized and smaller species of Tityus; however, they are shorter than those previously observed in the larger species of the genus. Morphometric growth values of the different instars are smaller than those of other known species ofTityus. Keywords: Scorpiones, Tityus melanostictus, Trinidad, forest, life history. Introduction Tityus melanostictus Pocock, 1893 was originally described from Trinidad, without reference to a precise locality (Pocock 1893, Lourengo & Eickstedt 1987). This species has a restricted area of distribution limited to Trinidad, Tobago and the North of Venezuela (Lourengo & Eickested 1987, Gonzalez-Sponga 1996, Prendini 2001). -

Virology Techniques
Chapter 5 - Lesson 4 Virology Techniques Introduction Virology is a field within microbiology that encom- passes the study of viruses and the diseases they cause. In the laboratory, viruses have served as useful tools to better understand cellular mechanisms. The purpose of this lesson is to provide a general overview of laboratory techniques used in the identification and study of viruses. A Brief History In the late 19th century the independent work of Dimitri Ivanofsky and Martinus Beijerinck marked the begin- This electron micrograph depicts an influenza virus ning of the field of virology. They showed that the agent particle or virion. CDC. responsible for causing a serious disease in tobacco plants, tobacco mosaic virus, was able to pass through filters known to retain bacteria and the filtrate was able to cause disease in new plants. In 1898, Friedrich Loef- fler and Paul Frosch applied the filtration criteria to a disease in cattle known as foot and mouth disease. The filtration criteria remained the standard method used to classify an agent as a virus for nearly 40 years until chemical and physical studies revealed the structural basis of viruses. These attributes have become the ba- sis of many techniques used in the field today. Background All organisms are affected by viruses because viruses are capable of infecting and causing disease in all liv- ing species. Viruses affect plants, humans, and ani- mals as well as bacteria. A virus that infects bacteria is known as a bacteriophage and is considered the Bacteriophage. CDC. Chapter 5 - Human Health: Real Life Example (Influenza) 1 most abundant biological entity on the planet. -

Interpretation of Serologic Tests
COCCIDIOIDOMYCOSIS SEROLOGY LABORATORY School of Medicine, University of California, Davis, California, 95616-8645 George Thompson M.D., Director NPI: 1831206812 VETERINARY CLIENTS Application and Interpretation of Serologic Tests A. Our usual serologic tests for coccidioidomycosis are the following: 1. Qualitative: Immunodiffusion (ID) to determine presence of coccidioidal IgM ("precipitin") (test may be termed “IDTP”), or IgG (CF) (test may be termed “IDCF”) antibody in body fluids. This is appropriate if no diagnosis of coccidioidomycosis has previously been established. [Non-specific reactions resembling that given by the coccidioidal precipitin (IgM) are encountered infrequently with some sera, e.g. from patients who do not have coccidioidomycosis.] 2. Quantitative: For veterinary specimens, we estimate the titer of the CF (IgG) by quantitative immunodiffusion rather than by complement fixation (CF). This provides an approximation of the CF titer. The quantitative immunodiffusion rather than the CF test avoids the problem of anticomplementary activity frequently encountered especially with canine sera. Determination of titer is appropriate after a diagnosis has been established, e.g. by positive immunodiffusion or recovery of C. immitis from the patient. B. Results and interpretation: 1. Detection of coccidioidal antibody in a single specimen can be diagnostically significant; testing sequential samples can be helpful in following the course of the disease. 2. Immunodiffusion (ID) (qualitative) - presence or not of precipitin (IgM) or CF (IgG) antibody. The IgM is important in the diagnosis of acute primary coccidioidomycosis, and is detectable in most patients in 1 to 2 weeks after onset of symptoms, may persist several months, occasionally longer in association with a pulmonary cavity or disseminated disease. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -
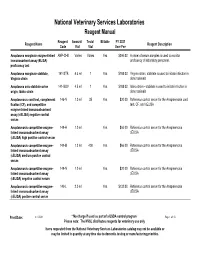
NVSL Reagent Manual
National Veterinary Services Laboratories Reagent Manual Reagent Amount/ Tests/ Billable FY 2021 Reagent Name Reagent Description Code Vial Vial User Fee Anaplasma marginale enzyme-linked ANP-CHK Varies VariesYes $394.00 A panel of serum samples is used to monitor immunosorbent assay (ELISA) proficiency of laboratory personnel. proficiency test Anaplasma marginale stabilate, 141-STB 4.5 ml 1Yes $188.00 Virginia strain, stabilate is used to initiate infection in Virginia strain donor animals Anaplasma ovis stabilate ovine 141-SOV 4.5 ml 1Yes $188.00 Idaho strain - stabilate is used to initate infection in origin, Idaho strain donor animals Anaplasmosis card test, complement 146-N 1.0 ml 35Yes $20.00 Reference control serum for the Anaplasmosis card fixation (CF), and competitive test, CF, and cELISA enzyme-linked immunoabsorbent assay (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-H 1.0 mlYes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) high positive control serum Anaplasmosis competitive enzyme- 149-M 1.0 ml 400Yes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) medium positve control serum Anaplasmosis competitive enzyme- 149-N 1.0 mlYes $20.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-L 2.0 mlYes $132.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) positve control serum Print Date: 6/11/2021 * No charge if used as part of a USDA control program Page 1 of 58 Please note: The NVSL distributes reagents for veterinary use only Items requested from the National Veterinary Services Laboratories catalog may not be available or may be limited in quantity at any time due to domestic testing or manufacturing priorities. -

Scorpions and Life-History Strategies: from Evolutionary Dynamics Toward the Scorpionism Problem Wilson R
Lourenço Journal of Venomous Animals and Toxins including Tropical Diseases (2018) 24:19 https://doi.org/10.1186/s40409-018-0160-0 REVIEW Open Access Scorpions and life-history strategies: from evolutionary dynamics toward the scorpionism problem Wilson R. Lourenço Abstract This work aims to contribute to the general information on scorpion reproductive patterns in general including species that can be noxious to humans. Scorpions are unusual among terrestrial arthropods in several of their life- history traits since in many aspects their reproductive strategies are more similar to those of superior vertebrates than to those of arthropods in general. This communication focuses mainly on the aspects concerning embryonic and post-embryonic developments since these are quite peculiar in scorpions and can be directly connected to the scorpionism problem. As in previous similar contributions, the content of this communication is addressed mainly to non-specialists whose research embraces scorpions in several fields such as venom toxins and public health. A precise knowledge of reproductive strategies presented by several scorpion groups and, in particular, those of dangerous species may prove to be a useful tool in the interpretation of results dealing with scorpionism, and also lead to a better treatment of the problems caused by infamous scorpions. Keywords: Scorpion, Reproductive strategies, Embryonic, Postembryonic development Background aspects of scorpion ecology and ecophysiology remain In a series of previous publications addressed to the incompletely studied but will not be the subject of the readers of the Journal of Venomous Animals and Toxins present communication. Contrarily, many reproductive including Tropical Diseases, I attempted to provide some aspects of scorpions are presently known and attest to general information about scorpions and scorpionism, the strong particularities in their mode of reproduction broadly addressed to non-specialists whose research em- [7]. -

A Hemagglutinin Quantification Method for Development of an Influenza Pandemic Vaccine Using Size Exclusion High Performance Liquid Chromatography
MOLECULAR MEDICINE REPORTS 11: 2819-2824, 2015 A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography HANG SIK ROH1, HYE MIN SONG1, BO REUM YUN1, HYUN KYUNG KANG1, KEUM SUK CHOI1, YUN JU PARK1, DONG SUB KIM1, SEUNG HEE KIM1, IN PIL MO2, BEUM‑SOO AN3 and CHI YOUNG AHN1 1Biologics Research Division, National Institute of Food and Drug Safety Evaluation, Korea Food and Drug Administration, Cheongwon‑gun, Chungcheongbuk‑do 363‑951; 2College of Veterinary Medicine, Chungbuk National University, Cheongju‑si, Chungcheongbuk‑do 361‑763; 3Department of Biomaterials Science, College of Natural Resources and Life Science, Pusan National University, Miryang, Gyeongsangnam‑do 627‑706, Republic of Korea Received February 17, 2014; Accepted November 19, 2014 DOI: 10.3892/mmr.2014.3049 Abstract. Single radial immunodiffusion (SRID) assay calculation based on SE‑HPLC exhibited 99.91‑100% simi- requires a reference antigen and an antibody to the hemag- larity, compared with that of SRID. These findings suggest glutinin (HA) of an influenza vaccine. As it takes 2‑3 months that the measurement of peak area ratio/HA content using to develop the reference antigen, vaccine development is SE‑HPLC may be a substitute for SRID and rapidly measure delayed in cases of an influenza pandemic. In the present HA content to enable faster development of a vaccine during study, the measurement of the HA content of influenza an influenza pandemic. vaccines was assessed using size exclusion high performance liquid chromatography (SE‑HPLC) for the rapid develop- Introduction ment of a pandemic vaccine.