Hydrophilic Linkers and Their Uses for Conjugation of Drugs to Cell Binding Molecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis and Biological Evaluation of Trisindolyl-Cycloalkanes and Bis- Indolyl Naphthalene Small Molecules As Potent Antibacterial and Antifungal Agents
Synthesis and Biological Evaluation of Trisindolyl-Cycloalkanes and Bis- Indolyl Naphthalene Small Molecules as Potent Antibacterial and Antifungal Agents Dissertation Zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) Vorgelegt der Naturwissenschaftlichen Fakultät I Institut für Pharmazie Fachbereich für Pharmazeutische Chemie der Martin-Luther-Universität Halle-Wittenberg von Kaveh Yasrebi Geboren am 09.14.1987 in Teheran/Iran (Islamische Republik) Gutachter: 1. Prof. Dr. Andreas Hilgeroth (Martin-Luther-Universität Halle-Wittenberg, Germany) 2. Prof. Dr. Sibel Süzen (Ankara Üniversitesi, Turkey) 3. Prof. Dr. Michael Lalk (Ernst-Moritz-Arndt-Universität Greifswald, Germany) Halle (Saale), den 21. Juli 2020 Selbstständigkeitserklärung Hiermit erkläre ich gemäß § 5 (2) b der Promotionsordnung der Naturwissenschaftlichen Fakultät I – Institut für Pharmazie der Martin-Luther-Universität Halle-Wittenberg, dass ich die vorliegende Arbeit selbstständig und ohne Benutzung anderer als der angegebenen Hilfsmittel und Quellen angefertigt habe. Alle Stellen, die wörtlich oder sinngemäß aus Veröffentlichungen entnommen sind, habe ich als solche kenntlich gemacht. Ich erkläre ferner, dass diese Arbeit in gleicher oder ähnlicher Form bisher keiner anderen Prüfbehörde zur Erlangung des Doktorgrades vorgelegt wurde. Halle (Saale), den 21. Juli 2020 Kaveh Yasrebi Acknowledgement This study was carried out from June 2015 to July 2017 in the Research Group of Drug Development and Analysis led by Prof. Dr. Andreas Hilgeroth at the Institute of Pharmacy, Martin-Luther-Universität Halle-Wittenberg. I would like to thank all the people for their participation who supported my work in this way and helped me obtain good results. First of all, I would like to express my gratitude to Prof. Dr. Andreas Hilgeroth for providing me with opportunity to carry out my Ph.D. -

Estimated Burden of Serious Fungal Infections in Ghana
Journal of Fungi Article Estimated Burden of Serious Fungal Infections in Ghana Bright K. Ocansey 1, George A. Pesewu 2,*, Francis S. Codjoe 2, Samuel Osei-Djarbeng 3, Patrick K. Feglo 4 and David W. Denning 5 1 Laboratory Unit, New Hope Specialist Hospital, Aflao 00233, Ghana; [email protected] 2 Department of Medical Laboratory Sciences, School of Biomedical and Allied Health Sciences, College of Health Sciences, University of Ghana, P.O. Box KB-143, Korle-Bu, Accra 00233, Ghana; [email protected] 3 Department of Pharmaceutical Sciences, Faculty of Health Sciences, Kumasi Technical University, P.O. Box 854, Kumasi 00233, Ghana; [email protected] 4 Department of Clinical Microbiology, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi 00233, Ghana; [email protected] 5 National Aspergillosis Centre, Wythenshawe Hospital and the University of Manchester, Manchester M23 9LT, UK; [email protected] * Correspondence: [email protected] or [email protected] or [email protected]; Tel.: +233-277-301-300; Fax: +233-240-190-737 Received: 5 March 2019; Accepted: 14 April 2019; Published: 11 May 2019 Abstract: Fungal infections are increasingly becoming common and yet often neglected in developing countries. Information on the burden of these infections is important for improved patient outcomes. The burden of serious fungal infections in Ghana is unknown. We aimed to estimate this burden. Using local, regional, or global data and estimates of population and at-risk groups, deterministic modelling was employed to estimate national incidence or prevalence. Our study revealed that about 4% of Ghanaians suffer from serious fungal infections yearly, with over 35,000 affected by life-threatening invasive fungal infections. -

Fundamental Medical Mycology Errol Reiss
Fundamental Medical Mycology Fundamental Medical Mycology Errol Reiss Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia H. Jean Shadomy Department of Microbiology and Immunology, Virginia Commonwealth University, School of Medicine, Richmond, Virginia G. Marshall Lyon, III Department of Medicine, Division of Infectious Diseases, Emory University, School of Medicine, Atlanta, Georgia A JOHN WILEY & SONS, INC., PUBLICATION This book was written by Errol Reiss in his private capacity. No official support or endorsement by the Centers for Disease Control and Prevention, Department of Health and Human Services is intended, nor should be inferred. Copyright 2012 by Wiley-Blackwell. All rights reserved Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4470, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permission. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. -

Novel Antimicrobial Agents Inhibiting Lipid II Incorporation Into Peptidoglycan Essay MBB
27 -7-2019 Novel antimicrobial agents inhibiting lipid II incorporation into peptidoglycan Essay MBB Mark Nijland S3265978 Supervisor: Prof. Dr. Dirk-Jan Scheffers Molecular Microbiology University of Groningen Content Abstract..............................................................................................................................................2 1.0 Peptidoglycan biosynthesis of bacteria ........................................................................................3 2.0 Novel antimicrobial agents ...........................................................................................................4 2.1 Teixobactin ...............................................................................................................................4 2.2 tridecaptin A1............................................................................................................................7 2.3 Malacidins ................................................................................................................................8 2.4 Humimycins ..............................................................................................................................9 2.5 LysM ........................................................................................................................................ 10 3.0 Concluding remarks .................................................................................................................... 11 4.0 references ................................................................................................................................. -

Current Therapies for Chronic Hepatitis C
Southern Illinois University Edwardsville SPARK Pharmacy Faculty Research, Scholarship, and Creative Activity School of Pharmacy 1-2011 Current Therapies for Chronic Hepatitis C McKenzie C. Ferguson Southern Illinois University Edwardsville, [email protected] Follow this and additional works at: https://spark.siue.edu/pharmacy_fac Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Ferguson, McKenzie C., "Current Therapies for Chronic Hepatitis C" (2011). Pharmacy Faculty Research, Scholarship, and Creative Activity. 4. https://spark.siue.edu/pharmacy_fac/4 This Article is brought to you for free and open access by the School of Pharmacy at SPARK. It has been accepted for inclusion in Pharmacy Faculty Research, Scholarship, and Creative Activity by an authorized administrator of SPARK. For more information, please contact [email protected],[email protected]. Chronic Hepatitis C & Current Therapies 1 Review of Chronic Hepatitis C & Current Therapies Reviews of Therapeutics Pharmacotherapy McKenzie C. Ferguson, Pharm.D., BCPS From the School of Pharmacy, Southern Illinois University Edwardsville, Department of Pharmacy Practice, Edwardsville, Illinois. Address for reprint requests : Southern Illinois University Edwardsville School of Pharmacy 220 University Park Drive, Ste 1037 Edwardsville, IL 62026-2000 Email: [email protected] Keywords : hepatitis C, HCV, ribavirin, interferon, peginterferon alfa-2a, peginterferon alfa-2b, albinterferon, taribavirin, telaprevir, therapeutic efficacy, safety The author received no sources of support in the form of grants, equipment, or drugs. Chronic Hepatitis C & Current Therapies 2 ABSTRACT Hepatitis C virus affects more than 180 million people worldwide and as many as 4 million people in the United States. Given that most patients are asymptomatic until late in disease progression, diagnostic screening and evaluation of patients that display high-risk behaviors associated with acquisition of hepatitis C should be performed. -
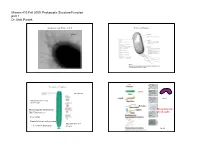
Parsek Micro410 Lecture
Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Organization of the Prokaryotic Cell Prokaryotic Structures fimbriae Size Range of Prokaryotes bacillus See Table 4.1 (rigid) vibrio Nanobacteria 0.05‐0.2 µm (0.14‐0.2 µm) (flexible) Thiomargarita namibiensis Mycoplasma are 700‐750 µm (Fig. 4.2) pleomorphic green alga Nanochlorum eukaryotum Mycoplasma 0.1‐ ~1-2 µm in diameter 0.3 µm Fig. 4.1 Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Staining cells for Microscopic observation Cell Arrangements Motility- ~80% of prokaryotes are motile streptococcus Staining properties: Gram Stain staphylococcus Fig. 2.3 Gram Stain (1884) (Bacteria) Gram-negative mixed culture Gram-positive Fig. 2.3 and 2.4 Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Functions of the cytoplasmic membrane The phospholipid bi‐layer Fig. 4.9 Fig. 4.4 What is the structure of bacterial phospholipids? Other components of the cytoplasmic membrane Figs. 4.5‐4.6 Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Archaeal membranes can be a lipid monolayer Archaeal phospholipids have an ether linkage Fig. 4.7 Fig. 4.8 Importance of Cell Wall Schematic diagram cell wall • Provides rigidity to cell allowing cell to withstand the large osmotic/ionic Fig. 4.16 changes a bacterium may experience in its environment, and turgor pressure of cytoplasm (conc. of solutes in cytoplasm). Cell lysis • May have a role in shape determination. • Provides a barrier against certain toxic chemical and biological agents. • Site of action of some of the most commonly used antibiotics used to treat bacterial infections (penicillin family). -
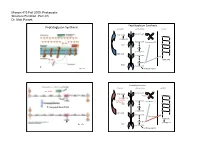
Parsek Lecture #3
Microm 410 Fall 2009: Prokaryotic Structure/Function: Part 2/3 Dr. Matt Parsek Peptidoglycan Synthesis Peptidoglycan Synthesis cytoplasm cell membrane cell wall Bactoprenol-P Pi UDP-NAM M G pentapeptide G M Bactoprenol Bactoprenol-P-P P M UMP G P NAM G M pentapeptide M G UDP-NAG Bactoprenol G P NAM‐NAG P NAM-NAG UMP pentapeptide Fig. 6.7a Interbridge peptide Peptidoglycan Synthesis Cross-linking of Peptidoglycan Strands cytoplasm cell membrane cell wall autolysins Bactoprenol-P Pi UDP-NAM Bacitracin M G pentapeptide G D-cycloserine Bactoprenol M (Oxamycin) Bactoprenol-P-P P M UMP G P Transpeptidase (FtsI) NAM G M pentapeptide M G UDP-NAG Bactoprenol G Vancomycin P NAM‐NAG P NAM-NAG pentapeptide UMP Fig. 6.7b pentapeptide Interbridge peptide Microm 410 Fall 2009: Prokaryotic Structure/Function: Part 2/3 Dr. Matt Parsek Cross-linking of Peptidoglycan Strands Antibiotic Resistance autolysins • Inactivate antibiotic β-lactamase (penicillinase) Clavulanic acid β-lactams Augmentin and Trimentin (combination of clavulanic acid and transpeptidase amoxicillin or ampicillin respectively) penicillins and cephalosporins lysozyme • Change chemistry of target site • Limit access of the antibiotic to target site Fig. 6.5 Cell Shape Determination • Modifications made to Peptidoglycan: ‐ lysozyme: Protoplasts/spheroplasts ‐ autolysins Bacillus subtilis ‐ endopeptidase Heliobacter pylori • Protein(s) may play a major role ‐ MreB protein Caulobacter crescentus ‐ MreB has homology to actin, a component of the cytoskeleton of eukaryotes. Shape determining protein‐ crescentin Fig. 6.4 Microm 410 Fall 2009: Prokaryotic Structure/Function: Part 2/3 Dr. Matt Parsek Cell Wall Gram-positive Bacteria intermediate filaments in the bacteria Caulobacter crescentus glycerol similar predicted structures of crescentin and intermediate filaments Fig. -

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

WO 2015/028850 Al 5 March 2015 (05.03.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/028850 Al 5 March 2015 (05.03.2015) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C07D 519/00 (2006.01) A61P 39/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, C07D 487/04 (2006.01) A61P 35/00 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, A61K 31/5517 (2006.01) A61P 37/00 (2006.01) HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KN, KP, KR, A61K 47/48 (2006.01) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (21) International Application Number: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, PCT/IB2013/058229 SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (22) International Filing Date: TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, 2 September 2013 (02.09.2013) ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant: HANGZHOU DAC BIOTECH CO., LTD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, [US/CN]; Room B2001-B2019, Building 2, No 452 Sixth TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Street, Hangzhou Economy Development Area, Hangzhou EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, City, Zhejiang 310018 (CN). -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Sporothrix Schenckii: ➢ Thermal Dimorphic
Subcutaneous mycoses ➢1-Mycetoma ➢2-Sporotrichosis ➢3-Chromoblastomycosis ➢4-Rhinosporidiosis ➢5- Lobomycosis ➢6- Entomophthoramycosis Sporotrichosis Rose gardener’s disease Chronic desease Agent Sporothrix schenckii: ➢ Thermal dimorphic ➢ In soil ➢ On decaying vegetation,plants,plant products (hay, straw, sphagnum moss), and a variety of animals (cats) ➢ less than 37° C (hyphal) ➢ 37° C (yeast) ➢ Sporothrix brasiliensis ➢ Sporothrix globosa Epidemiology ➢Worldwide ➢Tropical regions ➢Mexico ➢Brazile ➢France ➢USA Occupational disease: ➢Farmers ➢ ➢Workers ➢Gardeners ➢Florists Predisposing factors: ➢Trauma ➢Inhalation (very rarely) ➢HIV Clinical Syndromes 1-lymphocutaneous 2-Fixed cutaneous 3- Osteoarticular involvement 4-Pulmonary 5-Systemic Primary infection 1-Lymphocutaneous sporotrichosis 2-Fixed cutaneous sporotrichosis: Fixed cutaneous sporotrichosis verrucous-type sporotrichosis localized cutaneous type Paronychia sporotrichosis Osteoarticular involvement Pulmonary sporotrichosis: ➢Alcoholic ➢Pulmonary tuberculosis, diabetes mellitus and steroid ➢A productive cough ➢Low-grade fever ➢Weight loss Systemic sporotrichosis Transmission: ➢Dog bite ➢parrot bite ➢Insects bite ➢Cases of animal-to-human transmission Laboratory Diagnosis: 1-Collection of samples: ➢Drainage from skin lesions ➢Exudates ➢Pus ➢Blood ➢Pulmonary secretions ➢Tissue biopsy specimens 2-Direct examination ➢Gram ➢PAS ➢GMS ➢H & E ❖Yeast Cells ❖Asteroid body: Elongated Buds (“Cigar Body”) Wet Mount BHI Blood 37˚C Yeast with Elongated Daughter Cell Biopsy of subcutaneous tissue -

Histopathology of Important Fungal Infections
Journal of Pathology of Nepal (2019) Vol. 9, 1490 - 1496 al Patholo Journal of linic gist C of of N n e o p ti a a l- u i 2 c 0 d o n s 1 s 0 a PATHOLOGY A m h t N a e K , p d of Nepal a l a M o R e d n i io ca it l A ib ss xh www.acpnepal.com oc g E iation Buildin Review Article Histopathology of important fungal infections – a summary Arnab Ghosh1, Dilasma Gharti Magar1, Sushma Thapa1, Niranjan Nayak2, OP Talwar1 1Department of Pathology, Manipal College of Medical Sciences, Pokhara, Nepal. 2Department of Microbiology, Manipal College of Medical Sciences , Pokhara, Nepal. ABSTRACT Keywords: Fungus; Fungal infections due to pathogenic or opportunistic fungi may be superficial, cutaneous, subcutaneous Mycosis; and systemic. With the upsurge of at risk population systemic fungal infections are increasingly common. Opportunistic; Diagnosis of fungal infections may include several modalities including histopathology of affected tissue Systemic which reveal the morphology of fungi and tissue reaction. Fungi can be in yeast and / or hyphae forms and tissue reactions may range from minimal to acute or chronic granulomatous inflammation. Different fungi should be differentiated from each other as well as bacteria on the basis of morphology and also clinical correlation. Special stains like GMS and PAS are helpful to identify fungi in tissue sections. INTRODUCTION Correspondence: Dr Arnab Ghosh, MD Fungal infections or mycoses may be caused by Department of Pathology, pathogenic fungi which infect healthy individuals or by Manipal College of Medical Sciences, Pokhara, Nepal.