(Ceroplastes Destructor) and Chinese Wax Scale (C. Sinesis) (Hemi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ladybirds, Ladybird Beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-170 Ladybirds, Ladybird beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1 J. H. Frank R. F. Mizell, III2 Introduction Ladybird is a name that has been used in England for more than 600 years for the European beetle Coccinella septempunctata. As knowledge about insects increased, the name became extended to all its relatives, members of the beetle family Coccinellidae. Of course these insects are not birds, but butterflies are not flies, nor are dragonflies, stoneflies, mayflies, and fireflies, which all are true common names in folklore, not invented names. The lady for whom they were named was "the Virgin Mary," and common names in other European languages have the same association (the German name Marienkafer translates Figure 1. Adult Coccinella septempunctata Linnaeus, the to "Marybeetle" or ladybeetle). Prose and poetry sevenspotted lady beetle. Credits: James Castner, University of Florida mention ladybird, perhaps the most familiar in English being the children's rhyme: Now, the word ladybird applies to a whole Ladybird, ladybird, fly away home, family of beetles, Coccinellidae or ladybirds, not just Your house is on fire, your children all gone... Coccinella septempunctata. We can but hope that newspaper writers will desist from generalizing them In the USA, the name ladybird was popularly all as "the ladybird" and thus deluding the public into americanized to ladybug, although these insects are believing that there is only one species. There are beetles (Coleoptera), not bugs (Hemiptera). many species of ladybirds, just as there are of birds, and the word "variety" (frequently use by newspaper 1. -

Cambridge University Press 978-1-107-11607-8 — a Natural History of Ladybird Beetles M. E. N. Majerus , Executive Editor H. E. Roy , P
Cambridge University Press 978-1-107-11607-8 — A Natural History of Ladybird Beetles M. E. N. Majerus , Executive Editor H. E. Roy , P. M. J. Brown Index More Information Index 2-isopropyl-3-methoxy-pyrazine, 238 281, 283, 285, 287–9, 291–5, 297–8, 2-phenylethylamine, 237 301–3, 311, 314, 316, 319, 325, 327, 329, 335 abdomen, 17, 20, 22, 24, 28–9, 32, 38, 42, 110, Adalia 4-spilota,80 114, 125, 128, 172, 186, 189, 209–10, Adalia conglomerata, 255 218 adaline, 108, 237, 241 Acacia, 197, 199 adalinine, 237 acaricides, 316 adelgids, 29, 49, 62, 65, 86, 91, 176, 199, 308, Acaridae, 217 310, 322 Acarina, 205, 217 Adonia, 44, 71 Acer pseudoplatanus, 50, 68, 121 aggregations, 163, 165, 168, 170, 178, 184, Acraea, 228, 297, 302 221, 312, 324 Acraea encedana, 302 Aiolocaria, 78, 93, 133, 276 Acraea encedon, 297, 302 Aiolocaria hexaspilota,78 Acyrthosiphon nipponicum, 101 Aiolocaria mirabilis, 133, 276 Acyrthosiphon pisum, 75, 77, 90, 92, 97–101, albino, 273 116, 239 Alces alces,94 Adalia, 5–6, 10, 22, 34, 44, 64, 70, 78, 80, 86, Aleyrodidae, 91, 310 123, 125, 128, 130, 132, 140, 143, 147, alfalfa, 119, 308, 316, 319, 325 159–60, 166–7, 171, 180–1, 218, 222, alimentary canal, 29, 35, 221 234, 237, 239, 241, 255, 259–60, 262, alkaloids, x, 99–100, 195–7, 202, 236–9, 241–2, 269, 279, 281, 284, 286, 298, 311, 325, 245–6 327, 335 Allantonematidae, 220 Adalia 10-punctata, 22, 70, 80, 86, 98–100, anal cremaster, 38, 40 104, 108, 116, 132, 146–7, 149, Anatis, 4, 17, 23, 41, 44, 66, 76, 89, 102, 131, 154, 156, 160, 174, 181–3, 188, 148, 165, 186, 191, 193, -

Local and Regional Influences on Arthropod Community
LOCAL AND REGIONAL INFLUENCES ON ARTHROPOD COMMUNITY STRUCTURE AND SPECIES COMPOSITION ON METROSIDEROS POLYMORPHA IN THE HAWAIIAN ISLANDS A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI'I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY (ECOLOGY, EVOLUTION AND CONSERVATION BIOLOGy) AUGUST 2004 By Daniel S. Gruner Dissertation Committee: Andrew D. Taylor, Chairperson John J. Ewel David Foote Leonard H. Freed Robert A. Kinzie Daniel Blaine © Copyright 2004 by Daniel Stephen Gruner All Rights Reserved. 111 DEDICATION This dissertation is dedicated to all the Hawaiian arthropods who gave their lives for the advancement ofscience and conservation. IV ACKNOWLEDGEMENTS Fellowship support was provided through the Science to Achieve Results program of the U.S. Environmental Protection Agency, and training grants from the John D. and Catherine T. MacArthur Foundation and the National Science Foundation (DGE-9355055 & DUE-9979656) to the Ecology, Evolution and Conservation Biology (EECB) Program of the University of Hawai'i at Manoa. I was also supported by research assistantships through the U.S. Department of Agriculture (A.D. Taylor) and the Water Resources Research Center (RA. Kay). I am grateful for scholarships from the Watson T. Yoshimoto Foundation and the ARCS Foundation, and research grants from the EECB Program, Sigma Xi, the Hawai'i Audubon Society, the David and Lucille Packard Foundation (through the Secretariat for Conservation Biology), and the NSF Doctoral Dissertation Improvement Grant program (DEB-0073055). The Environmental Leadership Program provided important training, funds, and community, and I am fortunate to be involved with this network. -

San Jose Scale and Its Natural Enemies: Investigating Natural Or Augmented Controls
California Tree Fruit Agreement Research Report 2002 SAN JOSE SCALE AND ITS NATURAL ENEMIES: INVESTIGATING NATURAL OR AUGMENTED CONTROLS Project Leaders: Kent M. Daane Cooperators: Glenn Y. Yokota, Walter J. Bentley, Karen Sime, and Brian Hogg ABSTRACT San Jose scale (SJS) and its natural enemies were studied from 1999 through 2002. Natural populations were followed in stone fruit and almond blocks, with orchard management practices divided into “conventional” and “sustainable” practices, based on dormant and in-season insecticide use. Results generally show higher fruit damage at harvest-time in sustainably managed fields, although, these results are not consistent among orchards and exceptions to this pattern were found. In conventionally managed blocks, later harvest dates resulted in higher SJS fruit damage, although this did not hold true in sustainably managed orchards. Results from SJS pheromone-baited traps show a predominant seasonal pattern of SJS densities progressively increasing and parasitoid (Encarsia perniciosi) densities progressively decreasing. These data are discussed with respect to SJS fruit damage and parasitoid establishment and efficiency. SJS and parasitoid sampling methodology and distribution were investigated. Comparing SJS pheromone trap data to numbers of crawlers on double-sided sticky tape and SJS infested fruit at harvest show a significant correlation between pheromone trap counts of SJS males and numbers of SJS crawlers. Results suggest that there is a small window in the season (April-May) when sticky tape provides important information on crawler abundance and damage. Results show a negative correlation between the early season abundance of Encarsia (as measured by pheromone traps) and SJS damage at harvest. These results suggest that early-season ratios of parasitoid : SJS can not be used to predict fruit damage or biological control (these data require more analysis). -

Bionomics of Chilocorus Infernalis Mulsant, 1853 (Coleoptera
doi:10.14720/aas.2019.113.1.07 Original research article / izvirni znanstveni članek Bionomics of Chilocorus infernalis Mulsant, 1853 (Coleoptera: Coccinellidae), a predator of San Jose scale, Diaspidiotus perniciosus (Comstock, 1881) under laboratory conditions Razia RASHEED1*, A.A. BUHROO1 and Shaziya GULL1 Received July 23, 2018; accepted January 03, 2019. Delo je prispelo 23. julija 2018, sprejeto 03. januarja 2019. ABSTRACT IZVLEČEK The bionomics of Chilocorus infernalis Mulsant, 1853, a BIONOMIJA VRSTE Chilocorus infernalis Mulsant, 1853 natural enemy of San Jose scale, was studied under laboratory (Coleoptera: Coccinellidae), PLENILCA AMERIŠKEGA conditions (26 ± 2˚C, and 65 ± 5% relative humidity). The KAPARJA (Diaspidiotus perniciosus (Comstock, 1881)) V eggs were deposited in groups and on average 45.68 ± 24.70 LABORATORIJSKIH RAZMERAH eggs were laid by female. Mean observed incubation period was 6.33 ± 1.52 days. Four instar grubs were observed, and Bionomija vrste Chilocorus infernalis Mulsant, 1853, mean duration of all four grubs was found to be 19.98 days. naravnega sovražnika ameriškega kaparja, je bila preučevana The pupal stage lasted for 8.00 ± 0.50 days and after adults v laboratorijskih razmerah (26 ± 2˚C in 65 ± 5 % relativne emerged out. zračne vlažnosti). Samice plenilca so jajčeca odlagale v skupinah, v poprečju 45,68 ± 24,70 jajčec na samico. V Key words: bionomics; natural enemies; San Jose scale; povprečju so se ličinke razvile iz jajčec v 6,33 ± 1,52 dneh. incubation period; larval instars Ugotovljene so bile štiri larvalne stopnje, katerih povprečna življenska doba je bila 19,98 dni. Razvojni štadij bube je trajal 8,00 ± 0,50 dni, nakar so se izlegli imagi. -

COLEOPTERA COCCINELLIDAE) INTRODUCTIONS and ESTABLISHMENTS in HAWAII: 1885 to 2015
AN ANNOTATED CHECKLIST OF THE COCCINELLID (COLEOPTERA COCCINELLIDAE) INTRODUCTIONS AND ESTABLISHMENTS IN HAWAII: 1885 to 2015 JOHN R. LEEPER PO Box 13086 Las Cruces, NM USA, 88013 [email protected] [1] Abstract. Blackburn & Sharp (1885: 146 & 147) described the first coccinellids found in Hawaii. The first documented introduction and successful establishment was of Rodolia cardinalis from Australia in 1890 (Swezey, 1923b: 300). This paper documents 167 coccinellid species as having been introduced to the Hawaiian Islands with forty-six (46) species considered established based on unpublished Hawaii State Department of Agriculture records and literature published in Hawaii. The paper also provides nomenclatural and taxonomic changes that have occurred in the Hawaiian records through time. INTRODUCTION The Coccinellidae comprise a large family in the Coleoptera with about 490 genera and 4200 species (Sasaji, 1971). The majority of coccinellid species introduced into Hawaii are predacious on insects and/or mites. Exceptions to this are two mycophagous coccinellids, Calvia decimguttata (Linnaeus) and Psyllobora vigintimaculata (Say). Of these, only P. vigintimaculata (Say) appears to be established, see discussion associated with that species’ listing. The members of the phytophagous subfamily Epilachninae are pests themselves and, to date, are not known to be established in Hawaii. None of the Coccinellidae in Hawaii are thought to be either endemic or indigenous. All have been either accidentally or purposely introduced. Three species, Scymnus discendens (= Diomus debilis LeConte), Scymnus ocellatus (=Scymnobius galapagoensis (Waterhouse)) and Scymnus vividus (= Scymnus (Pullus) loewii Mulsant) were described by Sharp (Blackburn & Sharp, 1885: 146 & 147) from specimens collected in the islands. There are, however, no records of introduction for these species prior to Sharp’s descriptions. -

The Coccinellids (Coleoptera:Coccinellidae) Fauna in Manduwala Region, Dehradun, India
J. Exp. Zool. India Vol. 21, No. 1, pp. 177-183, 2018 www.connectjournals.com/jez ISSN 0972-0030 THE COCCINELLIDS (COLEOPTERA:COCCINELLIDAE) FAUNA IN MANDUWALA REGION, DEHRADUN, INDIA Dinesh Kumar Bhardwaj and Jyoti Falswal Department of Zoology, Dolphin (P.G.) Institute of Biomedical and Natural Sciences, Dehradun, India. (Accepted 17 October 2017) ABSTRACT : The latitude of Manduwala in Dehradun, Uttarakhand, India is 30.321915, and the longitude is 78.026619. Dehradun, Uttarakhand, India is located at India Country. These beetles are useful because of their predaceous Nature. The specimens of this research were collected by hand picking. These are of small size and easy to capture by hand. Killing Jar is a device used by entomologists to kill captured insects quickly and with minimum damage. The Jar, typically glass, must be hermetically sealable and one design has a thin layer of hardened plaster of paris on the bottom to absorb the killing agent. The killing agent will then slowly evaporate, allowing the jar to be used many times before needing to refresh the jar. A second method utilizes a wad of cotton placed in the bottom of jar. Liquid killing agent is then added until the absorbent material is nearly saturated. The most common killing agent is Chloroform and ethyl acetate. The species belong the family Coccinellidae; Sub family- Coccinellinae, the total of 9 species, Coccinella quinquepunctata, Coccinella hieroglyphica, Coccinella transversalis, Harmonia dimidiata, Coccinella leonine, Harmonia conformis, Coccinella septempunctata, Hippodamia veriegata, Halmus chalybeus was identified. Coccinellids are the most important and powerful predators of hemipteran pests including, aphids, mealybugs, scale insects and whiteflies. -

Coccidology. the Study of Scale Insects (Hemiptera: Sternorrhyncha: Coccoidea)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ciencia y Tecnología Agropecuaria (E-Journal) Revista Corpoica – Ciencia y Tecnología Agropecuaria (2008) 9(2), 55-61 RevIEW ARTICLE Coccidology. The study of scale insects (Hemiptera: Takumasa Kondo1, Penny J. Gullan2, Douglas J. Williams3 Sternorrhyncha: Coccoidea) Coccidología. El estudio de insectos ABSTRACT escama (Hemiptera: Sternorrhyncha: A brief introduction to the science of coccidology, and a synopsis of the history, Coccoidea) advances and challenges in this field of study are discussed. The changes in coccidology since the publication of the Systema Naturae by Carolus Linnaeus 250 years ago are RESUMEN Se presenta una breve introducción a la briefly reviewed. The economic importance, the phylogenetic relationships and the ciencia de la coccidología y se discute una application of DNA barcoding to scale insect identification are also considered in the sinopsis de la historia, avances y desafíos de discussion section. este campo de estudio. Se hace una breve revisión de los cambios de la coccidología Keywords: Scale, insects, coccidae, DNA, history. desde la publicación de Systema Naturae por Carolus Linnaeus hace 250 años. También se discuten la importancia económica, las INTRODUCTION Sternorrhyncha (Gullan & Martin, 2003). relaciones filogenéticas y la aplicación de These insects are usually less than 5 mm códigos de barras del ADN en la identificación occidology is the branch of in length. Their taxonomy is based mainly de insectos escama. C entomology that deals with the study of on the microscopic cuticular features of hemipterous insects of the superfamily Palabras clave: insectos, escama, coccidae, the adult female. -

Wax, Wings, and Swarms: Insects and Their Products As Art Media
Wax, Wings, and Swarms: Insects and their Products as Art Media Barrett Anthony Klein Pupating Lab Biology Department, University of Wisconsin—La Crosse, La Crosse, WI 54601 email: [email protected] When citing this paper, please use the following: Klein BA. Submitted. Wax, Wings, and Swarms: Insects and their Products as Art Media. Annu. Rev. Entom. DOI: 10.1146/annurev-ento-020821-060803 Keywords art, cochineal, cultural entomology, ethnoentomology, insect media art, silk 1 Abstract Every facet of human culture is in some way affected by our abundant, diverse insect neighbors. Our relationship with insects has been on display throughout the history of art, sometimes explicitly, but frequently in inconspicuous ways. This is because artists can depict insects overtly, but they can also allude to insects conceptually, or use insect products in a purely utilitarian manner. Insects themselves can serve as art media, and artists have explored or exploited insects for their products (silk, wax, honey, propolis, carmine, shellac, nest paper), body parts (e.g., wings), and whole bodies (dead, alive, individually, or as collectives). This review surveys insects and their products used as media in the visual arts, and considers the untapped potential for artistic exploration of media derived from insects. The history, value, and ethics of “insect media art” are topics relevant at a time when the natural world is at unprecedented risk. INTRODUCTION The value of studying cultural entomology and insect art No review of human culture would be complete without art, and no review of art would be complete without the inclusion of insects. Cultural entomology, a field of study formalized in 1980 (43), and ambitiously reviewed 35 years ago by Charles Hogue (44), clearly illustrates that artists have an inordinate fondness for insects. -

Beetles in a Suburban Environment: a New Zealand Case Study. The
tl n brbn nvrnnt: lnd td tl n brbn nvrnnt: lnd td h Idntt nd tt f Clptr n th ntrl nd dfd hbtt f nfld Alnd (4-8 GKhl . : rh At SI lnt rttn Mnt Albrt rh Cntr rvt Alnd lnd • SI lnt rttn prt • EW EAA EAME O SCIEIIC A IUSIA ESEAC 199 O Ο Ν Ε W Ε Ν ttr Grnt rd Τ Ε Ρ Ο Ι Ο Τ ie wi e suo o a oey Sciece eseac Ga om e ew eaa oey Gas oa is suo is gaeuy ackowege Ρ EW EAA SI ' EAME O lnt SCIEIIC A rttn IUSIA Wāhn ESEAC Mn p Makig Sciece Wok o ew eaa KUSCE G eees i a suua eiome a ew eaa case suy e ieiy a saus o Coeoea i e aua a moiie aias o yie Aucka (197-199 / G Kusce — Aucka SI 199 (SI a oecio eo ISS 11-1 ; o3 IS -77-59- I ie II Seies UC 5957(93111 © Cow Coyig uise y SI a oecio M Ae eseac Cee iae ag Aucka ew eaa eceme 199 ie y Geea iig Seices eso ew eaa Etiam pristina in aua Asο i a aua seig summa securitas et futura sweet tranquility and nature ., OISIECE e oe-eeig emoyci eee ioycus uuus (ou o is aie ooca os kaikaea (acycaus acyioies om e yie eee suey aea Aucka ew eaa e wie gaues o e eee ae oe cuses a ass ees is eee as a eic saus o uike a o e uaaa (Seoo as ossi eiece sows a e weei gou was iig i uassic imes way ack i e ea o e iosaus a gymosems moe a 1 miio yeas ago OEWO As a small boy in the 1930s I used to collect butterflies on the South Downs in southern England. -

Records of Ceroplastes Gray 1828 in Europe, with an Identification Key to Species in the Palaearctic Region
Bulletin of Insectology 65 (2): 291-295, 2012 ISSN 1721-8861 Records of Ceroplastes Gray 1828 in Europe, with an identification key to species in the Palaearctic Region Kinga FETYKÓ, Ferenc KOZÁR Department of Zoology, Plant Protection Institute, Centre for Agricultural Research, Hungarian Academy of Sciences, Budapest, Hungary Abstract The genus Ceroplastes (Hemiptera Coccoidea Coccidae) in Europe is reviewed. Surveys of Hungarian nurseries, greenhouses and flower shops found plants infested with Ceroplastes scale insects. Among them, Ceroplastes rubens Maskell 1893 was identified. Since there is constant invasion of European markets by species of Ceroplastes, a key is presented to separate the species cur- rently known to occur in the Palaearctic Region. Key words: wax scales, introduced pests, distribution, taxonomy, Palaearctic Region, Hungary, identification key. Introduction has been recorded also from China, Egypt, Japan and South Korea (Ben-Dov et al., 2012). The establishment of several invasive scale insects The aim of the present work was to study the Cero- (Hemiptera Coccoidea) has been reported in Europe in plastes species in Central Europe and to construct an the last years (Pellizzari and Germain, 2010; Pellizzari identification key to those known from the Palaearctic and Kozár, 2011; Fetykó and Szita, 2012). This has in- Region. creased the number of species established outdoors, in greenhouses and on house plants. The increase in the number of species of the genus Materials and methods Ceroplastes Gray 1828 (Hemiptera Coccoidea Coccidae Ceroplastinae) in the Palaearctic region is remarkable. A survey was conducted between 2006 and 2012 in According to the ScaleNet database (Ben-Dov et al., some Central European greenhouses, botanical gardens, 2012) 13 species have been recorded from the Palaearc- nurseries and on house plants; 75 samples of scale in- tic Region, 8 of them from Europe. -
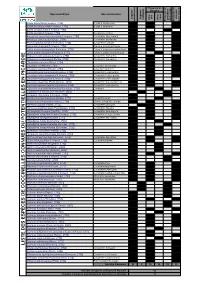
Liste Des Coccinelles De Picar
t s e n Présence en n a e e e d s o i s s s n i n e Picardie r e e e e e c g o i é u c c é c c l t é h q - è è n n n - r i h C e Nom scientifique Nom vernaculaire p t o e e p p n c u i e t a u l t s o r s s l s s l o o s q c x t n é i é é e i E E d e r r e a r u p A H d P P a d Adalia bipunctata (Linnaeus, 1758) Adalie à deux points 1 1 1 Adalia decempunctata (Linnaeus, 1758) Adalie à dix points 1 1 1 Adalia conglomerata (Linnaeus, 1758) 1 1 Anatis ocellata (Linnaeus, 1758) Coccinelle ocellée 1 1 1 Anisosticta novemdecimpunctata (Linnaeus, 1758) Coccinelle des roseaux 1 1 1 Aphidecta obliterata (Linnaeus, 1758) Coccinelle de l'épicea 1 1 1 Brumus quadripustulatus (Linnaeus, 1758) Coccinelle à virgule 1 1 1 Calvia decemguttata (Linnaeus, 1758) Calvia à dix points blancs 1 1 1 Calvia quatuordecimguttata (Linnaeus, 1758) Calvia à quatorze points blancs 1 1 1 Calvia quindecimguttata (Fabricius, 1777) Calvia à quinze points blancs 1 1 E Chilocorus bipustulatus (Linnaeus, 1758) Coccinelle des landes 1 1 1 I Chilocorus renipustulatus (Scriba, 1790) Coccinelle des saules 1 1 1 D Clitostethus arcuatus (Rossi, 1794) 1 1 1 R Coccidula rufa (Herbst, 1783) Coccidule des marais 1 1 1 A Coccidula scutellata (Herbst, 1783) Coccidule tachetée 1 1 1 C I Coccinella septempunctata (Linnaeus, 1758) Coccinelle à sept points 1 1 1 P Coccinella undecimpunctata (Linnaeus, 1758) Coccinelle à onze points 1 1 1 N Coccinella hieroglyphica (Linnaeus, 1758) Coccinelle à hiéroglyphe 1 1 E Coccinella magnifica (Redtenbacher, 1843) Coccinelle des fourmilières 1 1 1 Coccinella