University of Cape Town
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNEP/CBD/RW/EBSA/SIO/1/4 26 June 2013
CBD Distr. GENERAL UNEP/CBD/RW/EBSA/SIO/1/4 26 June 2013 ORIGINAL: ENGLISH SOUTHERN INDIAN OCEAN REGIONAL WORKSHOP TO FACILITATE THE DESCRIPTION OF ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS Flic en Flac, Mauritius, 31 July to 3 August 2012 REPORT OF THE SOUTHERN INDIAN OCEAN REGIONAL WORKSHOP TO FACILITATE THE DESCRIPTION OF ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS1 INTRODUCTION 1. In paragraph 36 of decision X/29, the Conference of the Parties to the Convention on Biological Diversity (COP 10) requested the Executive Secretary to work with Parties and other Governments as well as competent organizations and regional initiatives, such as the Food and Agriculture Organization of the United Nations (FAO), regional seas conventions and action plans, and, where appropriate, regional fisheries management organizations (RFMOs), with regard to fisheries management, to organize, including the setting of terms of reference, a series of regional workshops, with a primary objective to facilitate the description of ecologically or biologically significant marine areas (EBSAs) through the application of scientific criteria in annex I of decision IX/20, and other relevant compatible and complementary nationally and intergovernmentally agreed scientific criteria, as well as the scientific guidance on the identification of marine areas beyond national jurisdiction, which meet the scientific criteria in annex I to decision IX/20. 2. In the same decision (paragraph 41), the Conference of the Parties requested that the Executive Secretary make available the scientific and technical data and information and results collated through the workshops referred to above to participating Parties, other Governments, intergovernmental agencies and the Subsidiary Body on Scientific, Technical and Technological Advice (SBSTTA) for their use according to their competencies. -

Flinders Island Tourism Plan
F L I N D E R S I S L A N D N A T U R E B A S E D T O U R I S M M A R K E T F E A S I B I L I T Y S T U D Y 1 5 J u n e , 2 0 1 0 Tourism and Recreation PO Box 837, Jindabyne NSW 2627 Planning Specialists [email protected] www.planningforpeople.com.au P 02 6456 2722 F 02 6456 2422 M 0402 152 613 CONTENTS 1. BACKGROUND .................................................................................. 4 1.1 Introduction .................................................................................. 4 1.2 Approach to project ........................................................................ 4 1.3 Links to Flinders Island Tourism Plan ................................................ 4 2. SITUATION ANALYSIS ....................................................................... 6 2.1 The island ..................................................................................... 6 2.2 The current nature based tourism offer ............................................. 6 2.3 The Current Visitor ......................................................................... 8 2.4 SWOT analysis ............................................................................. 10 2.5 The Major Challenges ................................................................... 12 3. THE TOURISM CONTEXT .................................................................. 13 3.1 Australian tourism trends .............................................................. 13 3.2 Social and environmental trends ................................................... -

Grey Petrels Returning to Campbell Island? Survey and Census 14 Years After Rodent Eradication
Parker et al. 2015 Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication Graham C. Parker, Kalinka Rexer-Huber, David Thompson Report to the Department of Conservation June 2015 __________________________________________________________________________________ Grey petrels, Campbell Island 1 Parker et al. 2015 Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication Report to the Department of Conservation Graham C. Parker 1*, Kalinka Rexer-Huber 1, 3, David Thompson 2 1 Parker Conservation, 126 Maryhill Terrace, Dunedin New Zealand 2 National Institute for Water and Atmosphere (NIWA), 301 Evans Bay Parade, Hataitai, Wellington New Zealand 3 Department of Zoology, University of Otago, 340 Great King Street, Dunedin New Zealand * Author for correspondence: [email protected] Please cite as: Parker, G.C., Rexer-Huber, K. and Thompson, D. 2015. Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication. Unpublished report to the Department of Conservation. Parker Conservation, Dunedin __________________________________________________________________________________ Grey petrels, Campbell Island 2 Parker et al. 2015 Contents Abstract ...................................................................................................................................... 3 Introduction ............................................................................................................................... -

An Assessment for Fisheries Operating in South Georgia and South Sandwich Islands
FAO International Plan of Action-Seabirds: An assessment for fisheries operating in South Georgia and South Sandwich Islands by Nigel Varty, Ben Sullivan and Andy Black BirdLife International Global Seabird Programme Cover photo – Fishery Patrol Vessel (FPV) Pharos SG in Cumberland Bay, South Georgia This document should be cited as: Varty, N., Sullivan, B. J. and Black, A. D. (2008). FAO International Plan of Action-Seabirds: An assessment for fisheries operating in South Georgia and South Sandwich Islands. BirdLife International Global Seabird Programme. Royal Society for the Protection of Birds, The Lodge, Sandy, Bedfordshire, UK. 2 Executive Summary As a result of international concern over the cause and level of seabird mortality in longline fisheries, the United Nations Food and Agricultural Organisation (FAO) Committee of Fisheries (COFI) developed an International Plan of Action-Seabirds. The IPOA-Seabirds stipulates that countries with longline fisheries (conducted by their own or foreign vessels) or a fleet that fishes elsewhere should carry out an assessment of these fisheries to determine if a bycatch problem exists and, if so, to determine its extent and nature. If a problem is identified, countries should adopt a National Plan of Action – Seabirds for reducing the incidental catch of seabirds in their fisheries. South Georgia and the South Sandwich Islands (SGSSI) are a United Kingdom Overseas Territory and the combined area covered by the Territorial Sea and Maritime Zone of South Georgia is referred to as the South Georgia Maritime Zone (SGMZ) and fisheries within the SGMZ are managed by the Government of South Georgia and South Sandwich Islands (GSGSSI) within the framework of the Convention on the Conservation of Antarctic Marine Living (CCAMLR). -

Procellaria Cinerea) on Antipodes Island, New Zealand
269 Notornis, 2013, Vol. 60: 269-278 0029-4470 © The Ornithological Society of New Zealand, Inc. Notes on the distribution, behaviour and status of grey petrel (Procellaria cinerea) on Antipodes Island, New Zealand ELIZABETH. A. BELL* PO Box 607, Blenheim, Marlborough 7240, New Zealand BRIAN D. BELL 35 Selmes Road, Rapaura, RD3, Blenheim 7273, New Zealand J. L. SIM 178 C.D. Farm Road, Ohau, New Zealand M.J. IMBER** 6 Hillcrest Lane, Levin 5500, New Zealand Abstract Aspects of the breeding biology of the grey petrel (Procellaria cinerea) were studied on Antipodes Island between April and June 2001. The island was surveyed to determine grey petrel distribution and four 2500 m2 census grids were established. The survey suggested that the distribution of grey petrels was restricted to steep, well-draining areas dominated by Poa litorosa tussock (approximately 510 ha of the 2025 ha island). Occupied burrow density within the 4 census grids ranged from 31 to 44 burrows (0.01 burrows per square metre). Extrapolating from the census grid density to the total grey petrel habitat resulted in a population estimate of 114,730 birds: 53,000 breeding pairs (range = 32,000- 73,000) and 8,670 non-breeding-birds (range = 4,000-16,320) were present on Antipodes Island. Aspects of the behaviour of the species were recorded. Comparisons are made with other members of the genus Procellaria. Bell, E.A.; Bell, B.D.; Sim, J.L.; Imber, M.J. 2013. Notes on the distribution, behaviour and status of grey petrel (Procellaria cinerea) on Antipodes Island, New Zealand. -

Group Foraging in Socotra Cormorants: a Biologging Approach to the Study of a Complex Behavior Timothée R
Group foraging in Socotra cormorants: A biologging approach to the study of a complex behavior Timothée R. Cook, Rob Gubiani, Peter G. Ryan, Sabir B. Muzaffar To cite this version: Timothée R. Cook, Rob Gubiani, Peter G. Ryan, Sabir B. Muzaffar. Group foraging in Socotra cormorants: A biologging approach to the study of a complex behavior. Ecology and Evolution, Wiley Open Access, 2017, 7 (7), pp.2025-2038. 10.1002/ece3.2750. hal-01526388 HAL Id: hal-01526388 https://hal.sorbonne-universite.fr/hal-01526388 Submitted on 23 May 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Received: 5 October 2016 | Revised: 6 December 2016 | Accepted: 22 December 2016 DOI: 10.1002/ece3.2750 ORIGINAL RESEARCH Group foraging in Socotra cormorants: A biologging approach to the study of a complex behavior Timothée R. Cook1,2 | Rob Gubiani3 | Peter G. Ryan2 | Sabir B. Muzaffar3 1Department of Evolutionary Ecology, Evolutionary Ecophysiology Abstract Team, Institute of Ecology and Environmental Group foraging contradicts classic ecological theory because intraspecific competition Sciences, University Pierre et Marie Curie, Paris, France normally increases with aggregation. -

A Review of the Abundance and Distribution of Striated Caracaras Phalcoboenus Australis on the Falkland Islands Micky Reeves &Am
A review of the abundance and distribution of Striated Caracaras Phalcoboenus australis on the Falkland Islands Aniket Sardana Micky Reeves & Sarah Crofts Falklands Conservation, May 2015 The authors dedicate this report to Mr. Ian Strange and Mr. Robin Woods whose earlier surveys laid much ground work. This work was funded by: Falklands Conservation is a company limited by guarantee in England & Wales #3661322 and Registered Charity #1073859. Registered as an Overseas Company in the Falkland Islands. Roy Smith “These birds, generally known among sealers by the name of “Johnny” rook, partake of the form and nature of the hawk and crow… Their claws are armed with large and strong talons, like those of an eagle; they are exceedingly bold and the most mischievous of all the feathered creation. The sailors who visit these islands, being often much vexed at their predatory tricks, have bestowed different names upon them, characteristic of their nature, as flying monkeys, flying devils….” Charles Bernard 1812‐13 “A tameness or lack of wariness is an example of the loss of defensive adaptations.... an ecological naiveté…these animals aren’t imbeciles. Evolution has merely prepared them for a life in a world that is simpler and more innocent”…. where humans are entirely outside their experience. David Quammen (Island Biography in an age of extinction) 1996 1 ABSTRACT The Falkland Islands are globally important for the Striated Caracaras (Phalcoboenus australis). They reside mainly on the outer islands of the archipelago in strong associated with seabird populations, and where human interference is relatively low. A survey of the breeding population conducted in the austral summers of 2013/2014 and 2014/2015 indicates that the current population is likely to be the highest it has been for perhaps the last 100 years. -
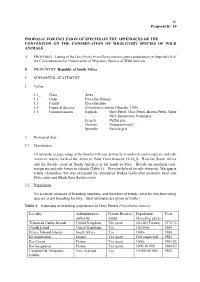
Grey Petrel Procellaria Cinerea (Entire Population) in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals
81 Proposal II / 10 PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A. PROPOSAL: Listing of the Grey Petrel Procellaria cinerea (entire population) in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals. B. PROPONENT: Republic of South Africa. C. SUPPORTING STATEMENT 1. Taxon 1.1_ Class Aves 1.2 Order Procellariiformes 1.3 Family Procellariidae 1.4 Genus & Species Procellaria cinerea (Gmelin, 1789) 1.5 Common names English: Grey Petrel, Gray Petrel, Brown Petrel, Great Grey Shearwater, Pediunker French: Puffin gris German: Grausturmvogel Spanish: Pardela gris 2. Biological data 2.1 Distribution Circumpolar pelagic range in the Southern Ocean, primarily in southern cool-temperate and sub- Antarctic waters north of the Antarctic Polar Front between 32-58S. Reaches South Africa and the Pacific coast of South America as far north as Peru. Breeds on southern cool- temperate and sub-Antarctic islands (Table 1). Previously bred on sub-Antarctic Macquarie Island (Australia), but was extirpated by introduced Wekas Gallirallus australis, feral cats Felis catus and Black Rats Rattus rattus. 2.2 Population No accurate censuses of breeding numbers, and therefore of trends, exist for this burrowing species at any breeding locality. Best estimates are given in Table 1. Table 1. Estimates of breeding populations of Grey Petrels Procellaria cinerea Locality Administrative Nature Reserve Population Year authority status (breeding pairs) Tristan da Cunha Islands United Kingdom Yes (part) 50-100 (Tristan) 1972/74 Gough Island United Kingdom Yes 100 000s 1984 Prince Edward Islands South Africa Yes 1000s 1984 Ile Amsterdam France Yes (part) Few suspected 1983 Iles Crozet France Yes (part) 1000s 1981/82 Iles Kerguelen France Yes (part) 5000-10 000 1984-87 Campbell & Antipodes New Zealand Yes 10 000-50 000 1984 Islands 82 Proposal II / 10 2.3 Habitat Marine, in southern coastal and pelagic waters. -

Overview of Tasmania's Offshore Islands and Their Role in Nature
Papers and Proceedings of the Royal Society of Tasmania, Volume 154, 2020 83 OVERVIEW OF TASMANIA’S OFFSHORE ISLANDS AND THEIR ROLE IN NATURE CONSERVATION by Sally L. Bryant and Stephen Harris (with one text-figure, two tables, eight plates and two appendices) Bryant, S.L. & Harris, S. 2020 (9:xii): Overview of Tasmania’s offshore islands and their role in nature conservation.Papers and Proceedings of the Royal Society of Tasmania 154: 83–106. https://doi.org/10.26749/rstpp.154.83 ISSN: 0080–4703. Tasmanian Land Conservancy, PO Box 2112, Lower Sandy Bay, Tasmania 7005, Australia (SLB*); Department of Archaeology and Natural History, College of Asia and the Pacific, Australian National University, Canberra, ACT 2601 (SH). *Author for correspondence: Email: [email protected] Since the 1970s, knowledge of Tasmania’s offshore islands has expanded greatly due to an increase in systematic and regional surveys, the continuation of several long-term monitoring programs and the improved delivery of pest management and translocation programs. However, many islands remain data-poor especially for invertebrate fauna, and non-vascular flora, and information sources are dispersed across numerous platforms. While more than 90% of Tasmania’s offshore islands are statutory reserves, many are impacted by a range of disturbances, particularly invasive species with no decision-making framework in place to prioritise their management. This paper synthesises the significant contribution offshore islands make to Tasmania’s land-based natural assets and identifies gaps and deficiencies hampering their protection. A continuing focus on detailed gap-filling surveys aided by partnership restoration programs and collaborative national forums must be strengthened if we are to capitalise on the conservation benefits islands provide in the face of rapidly changing environmental conditions and pressure for future use. -

Rapid Radiation of Southern Ocean Shags in Response to Receding Sea Ice 2 3 Running Title: Blue-Eyed Shag Phylogeography 4 5 Nicolas J
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.18.456742; this version posted August 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Rapid radiation of Southern Ocean shags in response to receding sea ice 2 3 Running title: Blue-eyed shag phylogeography 4 5 Nicolas J. Rawlence1, *, Alexander T. Salis1, 2, Hamish G. Spencer1, Jonathan M. Waters1, 6 Lachie Scarsbrook1, Richard A. Phillips3, Luciano Calderón4, Timothée R. Cook5, Charles- 7 André Bost6, Ludovic Dutoit1, Tania M. King1, Juan F. Masello7, Lisa J. Nupen8, Petra 8 Quillfeldt7, Norman Ratcliffe3, Peter G. Ryan5, Charlotte E. Till1, 9, Martyn Kennedy1,* 9 1 Department of Zoology, University of Otago, Dunedin, New Zealand. 10 2 Australian Centre for Ancient DNA, University of Adelaide, South Australia, Australia. 11 3 British Antarctic Survey, Natural Environment Research Council, United Kingdom. 12 4 Instituto de Biología Agrícola de Mendoza (IBAM, CONICET-UNCuyo), Argentina. 13 5 FitzPatrick Institute of African Ornithology, Department of Biological Sciences, University 14 of Cape Town, South Africa. 15 6 CEBC-CNRS, UMR 7372, 405 Route de Prissé la Charrière, 79360 Villiers en Bois, 16 France. 17 7 Justus Liebig University, Giessen, Germany. 18 8 Organisation for Tropical Studies, Skukuza, South Africa. 19 9 School of Human Evolution and Social Change, Arizona State University, Arizona, USA. 20 21 Prepared for submission as a research article to Journal of Biogeography 22 23 * Corresponding authors: [email protected]; [email protected] 24 25 ACKNOWLEDGEMENTS 26 This work was supported with funding from the University of Otago. -

Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds
Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds THREATENED SPECIES OCCASIONAL PUBLICATION NO. 17 Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds THREATENED SPECIES OCCASIONAL PUBLICATION NO. 17 by Graeme A. Taylor Published by Biodiversity Recovery Unit Department of Conservation PO Box 10-420 Wellington New Zealand Illustrations Front cover: Northern diving petrel, North Brothers Island, 1998 Inside front cover: Brown skua, Campbell Island, 1986 Source of illustrations All photographs were taken by the author unless stated otherwise. © May 2000, Department of Conservation ISSN 1170-3709 ISBN 0-478-21925-3 Cataloguing in Publication Taylor, Graeme A. Action plan for seabird conservation in New Zealand. Part B, Non-threatened seabirds / by Graeme A. Taylor. Wellington, N.Z. : Dept. of Conservation, Biodiversity Recovery Unit, 2000. 1. v. ; 30 cm. (Threatened Species occasional publication, 1170-3709 ; 17.) Cataloguing-in-Publication data. - Includes bibliographical references. ISBN 0478219253 1. Sea birds— New Zealand. 2. Rare birds—New Zealand. I. New Zealand. Biodiversity Recovery Unit. II. Title. Series: Threatened species occasional publication ; 17. 236 CONTENTS PART A: THREATENED SEABIRDS Abbreviations used in Parts A and B 7 Abstract 9 1 Purpose 11 2 Scope and limitations 12 3 Sources of information 12 4 General introduction to seabirds 13 4.1 Characteristics of seabirds 14 4.2 Ecology of seabirds 14 4.3 Life history traits of seabirds 15 5 New Zealand seabirds -

A Bulwer's Petrel Bulweria Bulwerii in Victoria
AUSTRALIAN 114 BIRD WATCHER AUSTRALIAN BIRD WATCHER 1989, 13, 114-117 A Bulwer's Petrel Bulweria bulwerii in Victoria by MIKE CARTER1 and TIM REID2 130 Canadian Bay Road, Mt Eliza, Victoria 3930 2Flat 2/61 Fawkner Street, St Kilda, Victoria 3182 Summary A Victorian record of Bulwer's Petrel Bulweria bulwerii is documented and discussed in relation to previous reports in the Australian region and vagrancy in the Atlantic. The observation A Bulwer's Petrel Bulweria bulwerii was seen at sea near Portland in south-western Victoria on 14 September 1986. It was flying among massed feeding seabirds at 38 ~l'S, 141 "34 'E, five nautical miles south-south-east of Cape Nelson. The sounder aboard the 12.8 m long fishing vessel engaged on an RAOU Seabird Group excursion indicated the depth of the ocean floor to be 75 m. Thus we were above the continental shelf in inshore waters. The bird was under observation for only a few minutes just before 0830 h E.S.T., but was well seen and scrutinised for diagnostic characters when it was realised that it was either one of the all-dark 'sooty' storm-petrels Oceanodroma spp. or a Bulwer's Petrel. The closest it approached the boat was about 50 m. The light was dull because of the heavily overcast skies but as the bird passed to the south and west of our position, the sun was behind us so viewing conditions were advantageous. Although the bird hugged the surface of the sea, it was only occasionally lost from view behind waves until it changed direction, headed away from the boat and disappeared.