1999 New Zealand Botanical Society
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Norton& Delange a Newsp.P65
NewNorton Zealand & de Lange—AJournal of newBotany, species 2003, of Vol.Coprosma 41: 223–231 223 0028–825X/03/4102–0223 $7.00 © The Royal Society of New Zealand 2003 A new species of Coprosma (Rubiaceae) from the South Island, New Zealand D. A. NORTON INTRODUCTION Conservation Research Group Coprosma is a Pacific Ocean-centred genus School of Forestry comprising some 110 species of trees and shrubs University of Canterbury (Oliver 1935; Van Balgooy 1966; Van Royen 1983; Private Bag 4800 Gardner 2002; Utteridge 2002). The genus is Christchurch, New Zealand distributed in temperate and montane-tropical Email: [email protected] regions from Borneo south-east to Australia, New P. J. de LANGE Zealand, and associated subantarctic islands, and Science & Research Unit across the Pacific Ocean to Hawai’i and Juan Auckland Conservancy Fernandez Islands. The centre of diversity for the Department of Conservation genus lies within New Zealand (Oliver 1935; Heads Private Bag 68908 1996), where there are c. 50–55 taxa formally Newton recognised (Eagle 1982; Dawson 2000; de Lange & Auckland, New Zealand Gardner 2002; de Lange et al. 2002) and at least a further seven entities awaiting formal recognition (cf. Eagle 1982). Abstract A new species, Coprosma fowerakeri, is One of these entities awaiting formal recognition, described from alpine habitats of the South Island, seemingly first recognised as distinct by the late New Zealand. Previously included within C. Tony Druce (1920–1999), as indicated by his pseudocuneata, it is distinguished by its low numerous collections in the Allan Herbarium (CHR), spreading habit; stout, recurved lateral branches that and variously known as Coprosma aff. -

Edition 2 from Forest to Fjaeldmark the Vegetation Communities Highland Treeless Vegetation
Edition 2 From Forest to Fjaeldmark The Vegetation Communities Highland treeless vegetation Richea scoparia Edition 2 From Forest to Fjaeldmark 1 Highland treeless vegetation Community (Code) Page Alpine coniferous heathland (HCH) 4 Cushion moorland (HCM) 6 Eastern alpine heathland (HHE) 8 Eastern alpine sedgeland (HSE) 10 Eastern alpine vegetation (undifferentiated) (HUE) 12 Western alpine heathland (HHW) 13 Western alpine sedgeland/herbland (HSW) 15 General description Rainforest and related scrub, Dry eucalypt forest and woodland, Scrub, heathland and coastal complexes. Highland treeless vegetation communities occur Likewise, some non-forest communities with wide within the alpine zone where the growth of trees is environmental amplitudes, such as wetlands, may be impeded by climatic factors. The altitude above found in alpine areas. which trees cannot survive varies between approximately 700 m in the south-west to over The boundaries between alpine vegetation communities are usually well defined, but 1 400 m in the north-east highlands; its exact location depends on a number of factors. In many communities may occur in a tight mosaic. In these parts of Tasmania the boundary is not well defined. situations, mapping community boundaries at Sometimes tree lines are inverted due to exposure 1:25 000 may not be feasible. This is particularly the or frost hollows. problem in the eastern highlands; the class Eastern alpine vegetation (undifferentiated) (HUE) is used in There are seven specific highland heathland, those areas where remote sensing does not provide sedgeland and moorland mapping communities, sufficient resolution. including one undifferentiated class. Other highland treeless vegetation such as grasslands, herbfields, A minor revision in 2017 added information on the grassy sedgelands and wetlands are described in occurrence of peatland pool complexes, and other sections. -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

A Synthesis of Geophysical and Biological Assessment to Determine Restoration Priorities
How can geomorphology inform ecological restoration? A synthesis of geophysical and biological assessment to determine restoration priorities A thesis submitted in partial fulfilment of the requirements for the Master of Science Degree in Physical Geography By Leicester Cooper School of Geography, Environment and Earth Sciences Victoria University Wellington 2012 _________________________________________________________________________________________________________________ Acknowledgements Many thanks and much appreciation go to my supervisors Dr Bethanna Jackson and Dr Paul Blaschke, whose sage advice improved this study. Thanks for stepping into the breach; I hope you didn’t regret it. Thanks to the Scholarships Office for the Victoria Master’s (by thesis) Scholarship Support award which has made the progression of the study much less stressful. Thanks go to the various agencies that have provided assistance including; Stefan Ziajia from Kapiti Coast District Council, Nick Page from Greater Wellington Regional Council, Eric Stone from Department of Conservation, Kapiti office. Appreciation and thanks go to Will van Cruchten, for stoically putting up with my frequent calls requesting access to Whareroa Farm. Also, many thanks to Prof. Shirley Pledger for advice and guidance in relation to the statistical methods and analysis without whom I might still be working on the study. Any and all mistakes contained within I claim sole ownership of. Thanks to the generous technicians in the Geography department, Andrew Rae and Hamish McKoy and fellow students, including William Ries, for assistance with procurement of aerial images and Katrin Sattler for advice in image processing. To Dr Murray Williams, without whose sudden burst at one undergraduate lecture I would not have strayed down the path of ecological restoration. -

Arachnid Ecology in New Zealand, Exploring
1 Arachnid ecology in New Zealand, exploring 2 unknown and poorly understood factors. 3 James Crofts-Bennett. 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 “A thesis submitted in fulfilment of the degree of Master of Science [1] in Botany [2] at the 21 University of Otago, Dunedin, New Zealand” 22 2020 23 1 24 Index 25 26 Abstract………………………………………………………………………………………5. 27 Chapter 1. Introduction……………………………………………………………………...7. 28 1.1 The importance of spiders………………………………………………………...7. 29 1.2 The influence of habitat structural complexity on spider distribution and 30 abundance…………………………………………………………………………......8. 31 1.3 Invasive rodents in the context of New Zealand Araneae………………………...9. 32 1.4 Thesis structure and aims………………………………………………………..14. 33 Chapter 2. The effect of habitat structural complexity on spider abundance and diversity..15. 34 2.1 Introduction ……………………………………………………………………..15. 35 Figure 2.1: Seasonal deciduous vegetation cover…………………………...16. 36 Figure 2.2: Seasonal deciduous vegetation cover with mistletoe parasites…16. 37 2.2 Methods…………………………………………………………………………17. 38 Figure 2.3: Examples of foliage samples……………………………………18. 39 Table 2.1: Sampling locations, dates and host data…………………………19. 40 2.2.1 Statistical Analyses……………………………………………………………20. 41 2.3 Results…………………………………………………………………………...20. 42 Figure 2.4: Total invertebrates sampled in summer, plotted………………..22. 43 Figure 2.5: Total invertebrates sampled in winter, plotted………………….23. 44 Table 2.2: Paired t-tests of host plant invertebrate populations……………..25. 45 2.4 Discussion……………………………………………………………………….26. 46 Chapter 3. A novel non-kill Araneae trap: test with regards to vegetation type versus 47 location 48 effects………………………………………………………………………………………..28. 49 3.1 Introduction……………………………………………………………………...28. -

Juncus Edgariae (Juncaceae) — a New Species from New Zealand
399 Juncus edgariae (Juncaceae) — a new species from New Zealand L.A.S. Johnson† and K.L. Wilson Abstract L.A.S. Johnson and K.L. Wilson. (Royal Botanic Gardens, Mrs Macquaries Road, Sydney NSW 2000, Australia) 2001. Juncus edgariae (Juncaceae) — a new species from New Zealand. Telopea 9(2): 399–402. The new species Juncus edgariae (in section Juncotypus) is described from New Zealand. It is allied to J. gregiflorus (Australia) and J. durus (New Guinea). Introduction The genus Juncus in Australasia was studied over many years by the first author, with assistance from the second author. The death of LASJ in 1997 left half-completed manuscripts of a revision of the genus in Australasia and Malesia and also a Flora of Australia treatment, which KLW is now completing. Publication of this new species in the ‘leafless’ (i.e. with leaves reduced to cataphylls) section Juncotypus (formerly known as section Genuini — Kirschner et al. 1999) is needed in advance of the main papers so that it can be included in the family treatment in the IOPI Species Plantarum Project’s Flora of the World series being completed by a team led by Jan Kirschner (Pruhonice). Juncus edgariae L.A.S. Johnson et K.L. Wilson, sp. nov. Affinis J. gregifloro sed culmis flavovirentibus nitentioribusque, medulla culmi densiori, minus interrupta, lacunis plusminusve ellipsoideis, capsulis fuscantioribus, differt. Type: New Zealand: South Island: S of Waimakariri River mouth, E. Edgar 8, 25 May 1960; holo NSW; iso CHR 113606. Strongly rhizomatous perennial. Culms terete, more or less hard (i.e. relatively difficult to compress), shining, yellow-green, 40–130 cm long, 0.6–2.5 mm diam.; striations 22–60; pith interrupted with irregular, more or less circular or longitudinally ellipsoidal gaps, occasionally continuous above. -

Assessing Pollination and Fruit Dispersal in Fuchsia Excorticata (Onagraceae)
RobertsonNew Zealand et al.—Pollination Journal of Botany, and 2008, dispersal Vol. in46 Fuchsia: 299–314 299 0028–825X/08/4603–0299 © The Royal Society of New Zealand 2008 Assessing pollination and fruit dispersal in Fuchsia excorticata (Onagraceae) ALastaIR W. ROBErtsON cases very frequently by silvereyes, which also oc- Ecology, Institute of Natural Resources casionally rob nectar from flowers. We confirmed Massey University that hermaphrodites account for more than half the Private Bag 11222 plants in all populations, are fully self-compatible, Palmerston North 4474, New Zealand and can autonomously self in the absence of pollina- [email protected] tors (especially in plants with smaller herkogamy). Fruit production in hermaphrodites and (particularly) JENNY J. LADLEY females was frequently pollen-limited (mean Pollen DAVE KELLY Limitation Indices of 0.17 and 0.40, respectively), School of Biological Sciences and was correlated with visual assessments of pol- University of Canterbury len loads on the stigma, a useful index of pollinator Private Bag 4800 service. A comparison of the proportion of ripe or Christchurch 8140, New Zealand overripe fruit on branches exposed to birds versus KatE L. MCNUTT branches enclosed in wire cages showed that un- Ecology, Institute of Natural Resources caged fruit on Kapiti Island is removed almost as Massey University soon as it is ripe but on the mainland it persists for Private Bag 11222 much longer. The proportion of ripe or overripe Palmerston North 4474, New Zealand compared to green fruit is therefore an approximate index of dispersal service. Both indices may be use- PAUL G. PETERSON ful to managers concerned with measuring the level Landcare Research of mutualism service provided by native birds. -
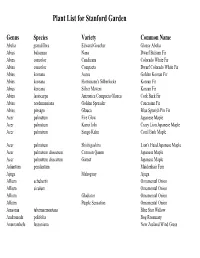
Plant List for Stanford Garden
Plant List for Stanford Garden Genus Species Variety Common Name Abelia grandiflora Edward Goucher Glossy Abelia Abies balsamea Nana Dwarf Balsam Fir Abies concolor Candicans Colorado White Fir Abies concolor Compacta Dwarf Colorado White Fir Abies koreana Aurea Golden Korean Fir Abies koreana Hortsmann's Silberlocke Korean Fir Abies koreana Silber Mavers Korean Fir Abies lasiocarpa Arizonica Compacta Glauca Cork Bark Fir Abies nordmanniana Golden Spreader Caucasian Fir Abies pinsapo Glauca Blue Spanish Pin Fir Acer palmatum Fire Glow Japanese Maple Acer palmatum Kurui Jishi Crazy Lion Japanese Maple Acer palmatum Sango Kaku Coral Bark Maple Acer palmatum Shishigashira Lion's Head Japanese Maple Acer palmatum dissectum Crimson Queen Japanese Maple Acer palmatum dissectum Garnet Japanese Maple Adiantum pendantum Maidenhair Fern Ajuga Mahogany Ajuga Allium schubertii Ornamental Onion Allium siculum Ornamental Onion Allium Gladiator Ornamental Onion Allium Purple Sensation Ornamental Onion Amsonia tabernaemontana Blue Star Willow Andromeda polifolia Bog Rosemary Anemanthele lessoniana New Zealand Wind Grass Genus Species Variety Common Name Anemone coronaria de Caen Wind Flower Anemone hupehensis japonica September Charm Japanese Anemone Anemone huphensis japonica Pamina Japanese Anemone Anemone x hybrida Honorine Jobert Japanese Anemone Angelica Aquilegia vulgaris Lime Frost Columbine Aquilegia Woodside Golden Columbine Aquillega Yellow Queen Yellow Columbine Arabis caucasica Variegata Variegated Rock Cress Arisaema formosanum DJHT 99049 Armeria maritima Alba White Sea Thrift Armeria maritima Dusseldorf Pride Sea Thrift Armeria pseudarmeria Arrhenatherum elatius ssp. Bulbosum Variegatum Striped Tuber Oat Grass Artemisia Oriental Limelinght Wormwood Artemisia Powis Castle Wormwood Arum italicum Wild Ginger Aruncus aethusifolius Dwarf Korean Goat's Beard Arundo donax Variegata Striped Giant Reed Arundo donax Giant Reed Asparagus densiflorus Myers Asparagus Fern/Pony Tail Fern Asplenium scolopendrium Hart's Tounge Fern Aster dumosus Prof A. -

Targeted Vegetation Survey of Floodplains and Lower Slopes on the Far North Coast © Department of Environment and Climate Change (NSW), 2008
Comprehensive Coastal Assessment September 2008 Targeted Vegetation Survey of Floodplains and Lower Slopes on the Far North Coast © Department of Environment and Climate Change (NSW), 2008 This document may not be re-produced without prior written permission from the Department of Environment and Climate Change (NSW). Department of Environment and Climate Change (NSW) 59-61 Goulburn Street (PO Box A290) Sydney South NSW 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131 555 (information & publications requests) TTY: (02) 9211 4723 Fax: (02) 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au Requests for information regarding this document are best directed to: Paul Sheringham Locked Bag 914 North East Branch Environmental Protection and Regulation Division Department of Environment and Climate Change Coffs Harbour NSW 2450 Phone: (02) 6659 8253 The documented may be cited as: Sheringham, P.R., Dr. Benwell, A., Gilmour, P., Graham, M.S., Westaway, J., Weber, L., Bailey, D., & Price, R. (2008). Targeted Vegetation Survey of Floodplains and Lower Slopes on the Far North Coast. A report prepared by the Department of Environment and Climate Change for the Comprehensive Coastal Assessment. Department of Environment and Climate Change (NSW), Coffs Harbour, NSW. Editing: P.J. Higgins. Design and layout: Dee Rogers ISBN 978 1 74122 857 1 DECC 2008/316 Printed on recycled paper CCA08 Far North Coast Targeted Vegetation Survey TARGETED VEGETATION SURVEY OF FLOODPLAINS AND LOWER SLOPES ON THE FAR NORTH COAST P.R. Sheringham, Dr. A. Benwell, P. Gilmour, M.S. Graham, J. Westaway, L. Weber, D. Bailey, & R. Price CCA08 SEPTEMBER 2008 CCA08 Far North Coast Targeted Vegetation Survey Credits Paul Sheringham: Botanist and project manager, and responsible for the survey and stratification of sites, data entry, numerical analysis and writing of this report. -

Breeding Systems and Reproduction of Indigenous Shrubs in Fragmented
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Breeding systems and reproduction of indigenous shrubs in fragmented ecosystems A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy III Plant Ecology at Massey University by Merilyn F Merrett .. � ... : -- �. � Massey University Palrnerston North, New Zealand 2006 Abstract Sixteen native shrub species with various breeding systems and pollination syndromes were investigated in geographically separated populations to determine breeding systems, reproductive success, population structure, and habitat characteristics. Of the sixteen species, seven are hermaphroditic, seven dioecious, and two gynodioecious. Two of the dioecious species are cryptically dioecious, producing what appear to be perfect, hermaphroditic flowers,but that functionas either male or female. One of the study species, Raukauaanomalus, was thought to be dioecious, but proved to be hermaphroditic. Teucridium parvifolium, was thought to be hermaphroditic, but some populations are gynodioecious. There was variation in self-compatibility among the fo ur AIseuosmia species; two are self-compatible and two are self-incompatible. Self incompatibility was consistent amongst individuals only in A. quercifolia at both study sites, whereas individuals in A. macrophylia ranged from highly self-incompatible to self-compatible amongst fo ur study sites. The remainder of the hermaphroditic study species are self-compatible. Five of the species appear to have dual pollination syndromes, e.g., bird-moth, wind-insect, wind-animal. High levels of pollen limitation were identified in three species at fo ur of the 34 study sites. -

Downloadable PDF Format On
Checklist of the New Zealand Flora Ferns and Lycophytes 2019 A New Zealand Plant Names Database Report © Landcare Research New Zealand Limited 2019 This copyright work is licensed under the Creative Commons Attribution 4.0 International license. Attribution if redistributing to the public without adaptation: "Source: Landcare Research" Attribution if making an adaptation or derivative work: "Sourced from Landcare Research" DOI: 10.26065/6s30-ex64 CATALOGUING IN PUBLICATION Checklist of the New Zealand flora : ferns and lycophytes [electronic resource] / Allan Herbarium. – [Lincoln, Canterbury, New Zealand] : Landcare Research Manaaki Whenua, 2017- . Online resource Annual August 2017- ISSN 2537-9054 I.Manaaki Whenua-Landcare Research New Zealand Ltd. II. Allan Herbarium. Citation and Authorship Schönberger, I.; Wilton, A.D.; Brownsey, P.J.; Perrie, L.R.; Boardman, K.F.; Breitwieser, I.; de Pauw, B.; Ford, K.A.; Gibb, E.S.; Glenny, D.S.; Korver, M.A.; Novis, P.M.; Prebble, J.M.; Redmond, D.N.; Smissen, R.D.; Tawiri, K. (2019) Checklist of the New Zealand Flora – Ferns and Lycophytes. Lincoln, Manaaki Whenua-Landcare Research. http://dx.doi.org/10.26065/6s30-ex64 This report is generated using an automated system and is therefore authored by the staff at the Allan Herbarium and collaborators who currently contribute directly to the development and maintenance of the New Zealand Plant Names Database (PND). Authors are listed alphabetically after the fourth author. Authors have contributed as follows: Leadership: Wilton, Breitwieser Database editors: Wilton, Schönberger, Gibb Taxonomic and nomenclature research and review for the PND: Schönberger, Wilton, Gibb, Breitwieser, Brownsey, de Lange, Ford, Fife, Glenny, Novis, Perrie, Prebble, Redmond, Smissen Information System development: Wilton, De Pauw, Cochrane Technical support: Boardman, Korver, Redmond, Tawiri Contents Introduction....................................................................................................................................................... -

Dryopteris Filix-Mas (L.) Schott, in Canterbury, New Zealand
An Investigation into the Habitat Requirements, Invasiveness and Potential Extent of male fern, Dryopteris filix-mas (L.) Schott, in Canterbury, New Zealand A thesis Submitted in partial fulfilment Of the requirements for the Degree of Masters in Forestry Science By Graeme A. Ure School of Forestry University of Canterbury June 2014 Table of Contents Table of Figures ...................................................................................................... v List of Tables ......................................................................................................... xi Abstract ................................................................................................................ xiii Acknowledgements .............................................................................................. xiv 1 Chapter 1 Introduction and Literature Review ............................................... 1 1.1 Introduction ................................................................................................................. 1 1.2 Taxonomy and ecology of Dryopteris filix-mas .......................................................... 2 1.2.1 Taxonomic relationships .................................................................................... 2 1.2.2 Life cycle ............................................................................................................... 3 1.2.3 Native distribution .............................................................................................. 4 1.2.4 Native