12 Days of Science at Christmas #12DOSAC @Oamc Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Save These Instructions Breast Milk
INSTRUCTIONS FOR USE The Breastmilk Bag is ideal for breastmilk storage.The special material of the bag is impermeable to the air and moisture and maintains the quality of the breastmilk.Heat-sealed seams prevent the bag from tearing or bursting. TECHNICAL DETAILS Capacity 150ml(oz) BREAST MILK BAG No-leak, easy-to-close zip-lock No-spill stand-up bottom Model:BBA15 Double-walled for long and safe breastmilk Storage. Pre-sterilized for hygiene & convenience STORING YOUR BREAST MILK To prevent the growth of the bacteria,breastmilk which is not to be used immediately must be refrigerated. Refrigerate expressed milk immediately(within 1 hour of expressing) If you are going to use the expressed milk to feed your baby within45 hours after expression,put the breast milk in the back area of the fridge where is coolest (4℃ or lower) Freeze refrigerated milk immediately(not more than 6 hours) for storage if it will not be use within next 48 hours. DO NOT fill storage container more than 3/4 full to give rom for expansion .if you are going to freezemilk.It is easiest to freeze milk in individual feed SAVE THESE INSTRUCTIONS quantites of 60-125ml(2-4 hours) Never top up refrigerated or frozen milk with fresh milk except you are going to use it to feed your baby within an hour NOTE: Refrigeration is the recommended method for storing breast milk because it preserves the natural immunity factors of the milk better than freezing BBA15-IM-090112 GUIDELINE FOR STORAGE TIME IMPORTANT! Method of milk storage Use within NEVER defrost or heat breast milk in microwave oven or a pan of boiling water DO NOT add fresh milk to already forzen or refrigerated milk for storage. -

Collecting and Storing Breastmilk During the COVID Pandemic
COLLECTING AND STORING BREASTMILK DURING THE COVID-19 PANDEMIC Breastmilk is the BEST source of nutrition for infants. It is very unlikely that COVID-19 will be passed through your breastmilk. The COVID-19 infection does not mean you have to stop breastfeeding. Just include respiratory protection when you are near your baby. The benefits of skin to skin and breastfeeding of breastmilk feeds outweighs the risks of transmission of COVID-19. Special Directions for COVID-19 Before Pumping During Pumping After Pumping Positive Mothers • Wash your hands for 20 seconds • Pump directly into the cleaned • Wash your hands for 20 seconds If you are positive or think you have and lather with soap and rinse bottle connected to the pump and lather with soap and rinse been exposed: in warm water. or new milk storage bag. in warm water. • Use a face covering when you • Dry them with a clean paper • Massage the breast during • Label the container with date of are breastfeeding or expressing towel. pumping if doing a single pump expression and name. your milk. • Use cleaned pump kits and session. • Check the milk containers/bags • Pay special attention to bottles or breast milk bags. • Pump both breasts for leakage. Do not keep units handwashing for 20 seconds Check for tears in the bag or simultaneously when/if you are with leaks and tears. and lather with soap and rinse chipping of the bottles. comfortable managing it. • Put the fresh milk container in a in warm water and dry them • Wipe high touch surfaces with • Fill the milk containers ¾ full. -
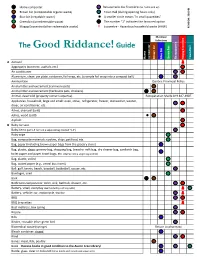
The Good Riddance!Guide
Home composter Ressourcerie des Frontières (at-home pick-up) Brown bin (compostable organic waste) Town Hall (during opening hours only) Blue bin (recyclable waste) A smaller circle means "in small quantities" Green bin (unredeemable waste) The number "1" indicates the favoured option Magog Ecocentre (other redeemable waste) Ecocentre - Hazardous household waste (HHW) REVISION: 2019-01 Municipal Collections The Good Riddance! Guide Home composter bin Brown bin Blue bin Green Ressourcerie Town Hall Magog Ecocentre Magog A Aerosol Aggregates (concrete, asphalt, etc.) Air conditioner Aluminium, clean: pie plate, container, foil wrap, etc. (crumple foil wrap into a compact ball) Ammunition Quebec Provincial Police Animal litter and excrement (carnivore pets) Animal litter and excrement (herbivore pets, chickens) Animal, dead wild (property owner's expense) Récupération Maillé 819 847-4907 Appliances, household, large and small: oven, stove, refrigerator, freezer, dishwasher, washer, dryer, air conditioner, etc. Ashes, charcoal (cold) Ashes, wood (cold) Asphalt B Baby car seat Baby items (put 0-5 items in a separate bag marked "0-5") Baby wipe Bag, composite materials: cookies, chips, pet food, etc. Bag, paper (including brown paper bags from the grocery store) Bag, plastic, clean: grocery bag, shopping bag, bread or milk bag, dry cleaner bag, sandwich bag, toilet paper and paper towel bags, etc. (Gather into a single bag and tie) Bag, plastic, soiled Bag, waxed paper (e.g., cereal box insert) Ball: golf, tennis, beach, baseball, basketball, -

Analysis of Milk Dispensers and Milk Cartons for Auburn School District
1 Analysis of Milk Dispensers and Milk Cartons for Auburn School District Prepared by King County Green Schools Program with support from City of Auburn (January 2021) Table of Contents Executive Summary Key Terms Summary of Benefits, Savings, and Costs Recommendations Comparative Analysis of Single-Use Milk Cartons and the Bulk Milk System Frequently Asked Questions on Bulk Milk System Implementation Summary of Resources Executive Summary Milk cartons represent a significant contribution to the solid waste generated by schools. Milk carton disposal presents challenges for custodians when students discard milk cartons that are not empty in garbage containers or recycling bins. Liquids in garbage or recycling collection bags can cause spillage when the bags are removed from collection bins, can increase the weight of bags for custodians who must lift the bags, and can create strong odors in dumpsters. When milk cartons with excess liquids are placed in outdoor recycling dumpsters or containers, excess liquid may result in contamination notices or extra fees from recycling haulers. In the Pacific Northwest, we know of two school districts that have implemented bulk milk systems in which bags of milk (from 3 to 5 gallons each) are placed in multi-spigot dispensers. The milk is served in durable cups which are washed after each use in school dishwashers along with other durable serving- ware such as trays. Olympia School District in Thurston County, Washington and Canby School District in Clackamas County, Oregon are using milk dispensers to reduce wasted milk, collection costs, and energy use. This report analyzes existing data on ASD milk carton purchases and solid waste and finds there is the potential for ASD to reduce costs by using a bulk milk system instead of single-use milk cartons. -

Looking Into Lactase a Medical Biotechnology Enzyme Lab
Looking Into Lactase A Medical Biotechnology Enzyme Lab Maryland Loaner Lab Teacher Packet Guided Inquiry Version ©Developed by Maryland High School teachers through a Dwight D. Eisenhower Professional Development Program. www.towson.edu/cse Version_8Aug19_KB Looking Into Lactase – Guided Table of Contents TEACHER MATERIALS Equipment and Supplies 2 Maryland Science Core Learning Goals 4 Setting Up the Lab 7 Solution Preparation and Aliquoting 7 Prepare 10 Student Workstations 8 Helpful Hints 8 Teacher Background 10 Teaching Scientific Inquiry: Explore Before Explain 10 Pre-Laboratory Activity: Teacher Guide 12 Laboratory Activity: Teacher Guide 13 Part 1: Identifying the Milk Type 13 Part 2: Designing Your Own Investigation 14 Interpretation of Results 16 Equipment Directions and Reagent Notes 18 Answers 19 Answers to Part 1 19 Answers to Part 2 22 Answers to Brief Constructed Response Questions 23 Answers to Extension Activities 24 Video Links 25 STUDENT ACTIVITY HANDOUTS AND LABORATORY PROTOCOL Medical Case Study S-1 Email #1 to Students S-3 Medical Fact Sheet #1 S-4 Email #2 to Students S-5 Student Laboratory Protocol – Part 1 S-6 Analysis Questions – Part 1 S-8 Student Laboratory Protocol – Part 2 S-9 Medical Fact Sheet #2 S-15 Assessment Questions S-16 Brief Constructed Response Questions S-16 Suggested Extension Activities S-18 1 | Page Looking Into Lactase – Guided Equipment and Supplies The Looking Into Lactase lab has two parts: • A pre-laboratory activity that allows students to learn about the relationship between lactose intolerance and enzyme function. • Two laboratory activities that allow students to first create their own protocol to identify three different milk types and then design their own investigation about how changes in conditions alter enzyme activity. -

Bulk Food & Reusable Containers List
MUNDARING Bulk Food & Reusable Containers List www.wastelesspantry.com.au Correct as at 24/4/19– New products arriving each week! Baking & Other .......... 1 Cream of Tartar Hemp Seeds Hulled Organic Beans & Seeds ........... 1 Custard Powder Lentils Green/Brown Beverages .................. 1 Epsom Salts Lentils Puy Bread Making ............ 2 Falafel Mix GF Lentils Red Cereals ....................... 2 Cleaning .................... 2 Flour Gluten Linseed Flaxseed Confectionary ........... 2 Gelatine Powder Linseed Flaxseed Meal Containers ................. 2 GF Baking Powder LSA Mix Dried Fruit ................... 2 GF Banana Bread Mix Peas Chick Flours ........................... 3 GF Muffin Mix Peas Split Green Liquids ......................... 3 Hundreds and Thousands Peas Split Yellow Reusables ................... 3 Maca Powder Organic Raw Pepitas Pumpkin Seed Organic Nuts ............................. 4 Pasta ........................... 4 MesQuite Powder Organic Popping Corn Organic (Aus) Personal Hygiene ...... 4 Milk Powder FC Poppy Seeds Pet Goods .................. 4 Pectin Sesame Seed Black Rice ............................. 4 Potato Starch Sesame Seeds Snacks ........................ 4 Protein Powder Pea 80% Sunflower Kernels (Aus) Sprayfree Spice & Seasonings ... 4 Savoury Nutritional Yeast Flakes Teff Sugar & Desserts ........ 5 Slippery Elm Bark Beverages Spinach Powder Baking & Other Coffee Bolt Blue Agar Agar TVP Mince Coffee Bolt Crack Bee Pollen Whey Protein Powder Coffee Bolt Organic Beetroot Powder Organic Xanthan -

20120613-153415.Pdf
KINGPAC COMPANY PROFILE WORLD CLASS PLASTIC BAGS MANUFACTURER KING PAC INDUSTRIAL CO.,LTD. THE LARGEST PLASTIC BAG MANUFACTURER IN ASIA SUPER MARKET INDUSTRIAL AGRICULTURAL GOVERNMENT INSTITUTIONS HOTEL AND CATERING HOUSE BRAND PRODUCTS THE LARGEST PLASTIC BAG MANUFACTURER IN ASIA INTRODUCTION The business world is moving more rapidly than ever. The plastic bag industry is one of the fastest moving segments among many industries today. Currently consumers are placing more emphasis on product images. Distributors must therefore move from price concerns to providing customer satisfaction and convenience for greater value creation. Being light in weight and easily transformable into various shapes and sizes, plastic bags are the answer for the needs of convenient packaging. Plastic packaging can also be applied to a variety of customer needs, both household and business use. Plastic Packaging is essentially a part of our daily life. Currently, KING PAC INDUSTRIAL CO., LTD is one of the leading plastic manufacturers in Asia with ambitious aims at becoming one of the world’s leading plastic bag manufacturers and exporters. With more than 20 years of business experience specializing in manufacturing of all types of plastic bags including vest carriers, shopping bags, fashion bags, garbage bags, zipper bags and many more. Our plastic bags are made from high quality Polyethylene (PE) at competitive prices with reliable delivery to customers worldwide. With professional expertise of our dedicated staff accompanied by advanced production technologies imported from the United States, Europe, and Japan, all of which has supported us in producing greater quality products. Our production capabilities have made KING PACK INDUSTRIAL CO., LTD the first and only manufacturer in Thailand with printing capabilities of up to 8 colours. -
Instructions for Use Instrucciones De Uso Easy
INSTRUCTIONS FOR USE INSTRUCCIONES DE USO EASY FREEZE BREAST MILK BAG BOLSA PARA LECHE MATERNA Ardo medical AG Gewerbestrasse 19 6314 Unterägeri Switzerland T +41(0)41 754 70 70 F +41(0)41 754 70 71 [email protected] www.ardo-usa.com 0123 Class 2a Distributed in USA by: Comit Developers 116 Toledo Drive Lafayette, LA 70506, USA T 844-411-ARDO (2736) www.ardo-usa.com [email protected] Art.No. 99.04.143 / 11.2014 Art.No. 1 2 3 4 5 6 7 8 9 10 a b c d e f g en INSTRUCTIONS FOR USE EASY FREEZE BREAST MILK BAG Package contains: 20 x Easy Freeze bag, 2 x fastening strip Intended use: Easy Freeze breast milk bags for easy and space-saving storage of breast milk in your fridge or freezer. a a) Perforated line 1 b c b) Eyelets for securing the fastening strip d c) Sterile pouch e d) Perforated line 2 f e) Temperature indicator f) Fastening strip g) Base of the bag g How to use Easy Freeze: - Note details of the date, amount (if necessary), etc. on the bag - If you only want to fill the Easy Freeze bag with breast milk, go to step (7) - Carefully separate the bag by tearing or cutting along perforated line 1 (1) - Undo the zip-fastener (2) - Position the Easy Freeze bag over the lower part of the breast shell (3) - Thread the fastening strip through both eyelets (4) - Fasten with the fastening strip (5) - Pump the breast milk, unfasten the bag and remove it from the lower part of the breast shell (6) - Remove the upper part of the bag by tearing or cutting along perforated line 2 (7) - Open out the folded base of the bag so it will stand by itself (8) - Gently press on the side of the bag to expel the air (9) - Zip the bag closed (10) - Keep the fastening strip to re-use it Storage of breast milk: At room temperature: 6 hours at max. -

Than a Nut Milk Bag Recipe Collection by Brenda Hinton
RAW FOODS COOKBOOK $15.00 US More than a NUT MILK BAG Rawsome Creations Recipe Collection More than a As a teacher of raw foods workshops, I get asked again and again, "What can I do with the NUT MILK BAG pulp after making nut milk?" I hate wasting good stuff, so I collected these recipes. After months in the kitchen and careful testing by a dedicated group of Recipe tasters, I am revealing the answer in this recipe collection! Over 40 tested and taster- approved recipes, from Collection beverages and snacks to main courses, are packed inside — a wealth of wonderful food ideas in by Brenda Hinton this slim volume! “this little book is so full of BIG ideas! Yum . and thank you” Rawsome Creations – Lara Moraga www.rawsomecreations.com UPC: Rawsome Creations More than a Nut Milk Bag Recipe Collection by Brenda Hinton Dear Reader, Thank you for looking at my collection of recipes. This place in a book is where you usually find praiseful words from famous people . but I don’t know any famous people, and anyway, this is not a book of, by, or for famous people. The simple fact that you have picked up my book and are reading these words means that you are exactly the kind of person whose feedback will be most valuable to me and future readers. So if you read this book, and find it useful (or find ways that you think I could make it more useful) I would like to hear from you. I am especially interested to know if you had problems with any of the recipes, or if I can make the directions clearer. -

Container Recycling Paper Recycling Information
Waste Management Information for Residents of North Algona Wilberforce and Sebastopol Ward of Bonnechere Valley Container recyclables must be placed loose inside All recycling, organic material and garbage must the Municipal Depot bins. Return all liquor, wine, be taken to your Municipal Depot. Complete beer containers purchased at the LCBO to the Beer waste management information and details Store for a refund. available on your Township’s Website. ✔ RECYCLABLES MUST BE EMPTY AND RINSED Hazardous and Electronic Waste accepted at the Ottawa Valley Waste Recovery Centre year round. Visit www.ovwrc.com. Aluminum cans & plates Aerosol (empty) Cardboard cans & tin cans INFORMATION Paper recycling must be placed loose inside the Municipal Depot bins. Cartons & drink boxes Egg cartons (plastic & white Styrofoam) Glass bottles & jars Cardboard (2 ft x 3ft (0.61 x 0.91 m), Plastic bags (ONLY fl attened and/or bread, produce, bundled) outside milk bag, blue Books (soft & hard cover- newspaper bags & remove hardcovers and grocery/retail bags- discard with garbage) Paint cans (METAL, stuff all bags into one) EMPTY/DRY, lids removed) NEW–WHITE ONLY Cereal, tissue, frozen Envelopes & Magazines, food, cracker boxes junk mail catalogues & phone books (remove Styrofoam – (*New – White plastic) Plastic bottles, jugs, ONLY) plates, cups, meat trays, tubs & lids rigid packaging. Remove plastic wrap & tape. No Styrofoam packing peanuts. Paper bags Plastic bakery, Newspapers & fl yers produce & clam shell (including glossy) Paper (colored & white) & Plastic food containers packaging fi le folders (yogurt, applesauce) DO NOT PUT IN RECYCLING ✘ Containers fi lled with liquid ✘ Medical waste Shredded paper (in Wrapping (non-metallic)/ Toilet & paper towel ✘ Sharps & Needles (dispose of at your local pharmacy) a clear plastic bag) tissue paper & greeting cards rolls (empty) CONTAINER PAPER RECYCLING RECYCLING Garbage must be placed in specially marked bags or bags To absorb moisture, reduce odours, and help keep with stickers adhered. -

Jai Jogeshwar Enterprises
+91-7971473000 Jai Jogeshwar Enterprises https://www.indiamart.com/jaijogeshwarenterprises-in/ We are famous for the Manufacturing of a wide assortment of Office Bags, Leather Bags, Food Delivery Bags, E-Commerce Delivery Bags, Milk Delivery Bag, Laptop Bag, Travel Bags, etc. These are popular for their strong stitching and superior quality. About Us Jai Jogeshwar Enterprises was commenced in the year 2018, with an affirmation to Manufacturing a wide array of Delivery Bag. Offered products range consists of Office Bags, Leather Bags, Food Delivery Bags, Travel Bags, Executive Document Bags, Gym Bag, Lunch Box Bag, Pizza Delivery Bag, Ladies College Bag, School and College Backpack, Milk Delivery Bag, Laptop Bag, E Commerce Delivery Bag etc. This offered bags range is constructed using ultra-modular technology at our state-of-the-art production unit. In addition to this, these bags are acclaimed for uncomplicated design, lightweight, easy to use, and longer service life. We have built a well-equipped infrastructure facility. With the valuable support of a skilled team of specialists, we carry out the processes of manufacturing to the delivery of quality approved bags in compliance with set industry measures. Our units are helped with contemporary tools and advanced technologies that are necessary for presenting an immaculate quality bags range at a rapid production rate. Our mentor Mr. Abhishek Rai maintains close relations in the company to offer bulk orders proficiently. He manages us to deliver exceptional quality office and leather -

Techy Tuesdays
Techy Tuesdays This weeks theme Fun at the fair! Miss Law’s Science activities at home Optical illusions can be found within funhouses and sometimes is used as part of sign designs at the fair. Here are some optical illusions you can have a look at yourself and with your family Are the horizontal lines sloping or straight? Are the circles moving in the image? Is the image spinning? https://www.optics4kids.org/illusions https://sciencebob.com/see-some-optical-illusions/ Bokeh effect photography Step 1 Set up your camera on a tripod or flat surface and set your camera's aperture to the widest setting, preferably f/1.8 or f/1.4 Step 2 Set up your fairy lights. Try to spread the lights evenly across the background you plan to use. Step 3 Set up your subject. Bokeh turns out better if you place the subject at least four to five feet in front of the lights. Step 4 If you are not using a fixed lens, zoom your lens in all the way. Step 5 Focus on your subject. Try to stay close to the subject, because if you are too away from it, your bokeh will suffer and appear smaller. Step 7 Release your camera's shutter, and voila! You have produced a photo with artificial bokeh obtained from simple fairy lights. https://photography.tutsplus.com/tutorials/quick-tip-creating-artificial-bokeh-in-your-own-home--photo-5773 Toffee Apples You will need:- •4 apples, Granny Smiths work well •200g caster sugar, golden if possible •½ tsp vinegar •2 tbsp golden syrup Place the apples in a large bowl, then carefully pour over some boiling water.