Analysis of Pyrotechnic Components
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From a Technical Standpoint, What Is Firework Flash Powder?
This article originally appeared in Fireworks Business, No. 246, 2004. From a Technical Standpoint, What Is Firework Flash Powder? K. L. Kosanke and L. Weinman perature attainable in pyrotechnic reactions, only Introduction solids and liquids incandesce, gases do not. As a In a recently published article on the regulatory practical matter, metal oxides are the only pyro- definitions of firework flash powder[1] it was con- technic reaction products that are not gaseous at cluded that none of those definitions provided suf- high temperature. Thus metal fuels need to be pre- ficient information to objectively establish wheth- sent in substantial quantity in flash powders. See er or not a pyrotechnic composition is a flash Table 1, which is a list of the boiling point of powder. That is to say, those definitions are all some of the pyrotechnic reaction products. The subjective to the extent that they depend on the table has somewhat arbitrarily been divided into intended use of the composition and none provide species with boiling points above and below a quantifiable measure that can be used to deter- 2500 °C. Note that the oxides of zirconium, mag- mine whether a particular pyrotechnic composi- nesium, aluminum and titanium top the list. tion is a flash powder. The purpose of the present article is to suggest a general approach that might Table 1. Boiling Point of Some Pyrotechnic be used as the basis for producing a quantitative [2] definition of flash powder. Reaction Products. The reason such an objectively quantifiable Reaction Product Boiling Point (°C) definition is needed is that – from both a regulato- ZrO2 ≈ 5000 ry and safety standpoint – flash powders are treat- MgO 3600 ed differently than other pyrotechnic composi- Al2O3 2980 tions. -

ENCYCLOPEDIA of CHEMISTRY & EXPLOSIVES MATERIALS Abelite
ENCYCLOPEDIA OF CHEMISTRY & EXPLOSIVES MATERIALS A Abelite An explosive, composed mainly of ammonium nitrate and trinitrotoluene. Absolute Zero The least possible temperature for all substances. Generally accepted as -273.15ÝC AC Alternating current. Acceptance Quality Level (AQL) A nominal value expressed in terms of percentage defective per hundred units, by which a group of sampling plans is identified. The sampling plans so identified have a high probability of accepting lots containing material with a process average not greater than the designed value of the AQL. Acetin [CH3COOC3H5(OH)2] also known as glyceryl monoacetate, a colourless hydroscopic liquid. Used as an intermediate for various explosives, and a solvent for various dyes. Acetone [CH3COCH3] colourless, flammable liquid. Acetone is widely used in industry as a solvent for many organic substances. It is used in making synthetic Resins and fillers, smokeless powders, and many other organic compounds. Boiling Point 56ÝC. Useful solvent for acetylene, also known as the simplest saturated ketone. Acetylene or ethyne, a colourless gas and the simplest alkyne Hydrocarbon. Explosive on contact with air, it is stored dissolved under pressure in Acetone. It is used to make neoprene rubber, plastics, and resins. The oxyacetylene torch mixes and burns oxygen and acetylene to produce a very hot flame-as high as 3480ÝC (6300ÝF)-that can cut steel and weld iron and other metals. Produced by the action of wateron calcium carbide and catalytically from naphtha. Acetylide A carbide formed by bubbling acetylene through a metallic salt solution, eg cuprous acetylide, Cu2C2. These are violently explosive compounds. Acid Any substance capable of giving up a proton; a substance that ionizes in solution to give the positive ion of the solvent; a solution with a pH measurement less than 7. -

Liothyronine Sodium(BANM, Rinnm) Potassium Perchlorate
2174 Thyroid and Antithyroid Drugs with methodological limitations. However, a controlled trial of In myxoedema coma liothyronine sodium may be liothyronine with paroxetine could not confirm any advantage of given intravenously in a dose of 5 to 20 micrograms by 3 O additive therapy. slow intravenous injection, repeated as necessary, usu- 1. Aronson R, et al. Triiodothyronine augmentation in the treat- HO I ally at intervals of 12 hours; the minimum interval be- ment of refractory depression: a meta-analysis. Arch Gen Psychi- OH atry 1996; 53: 842–8. tween doses is 4 hours. An alternative regimen advo- 2. Altshuler LL, et al. Does thyroid supplementation accelerate tri- NH2 cates an initial dose of 50 micrograms intravenously cyclic antidepressant response? A review and meta-analysis of I O the literature. Am J Psychiatry 2001; 158: 1617–22. followed by further injections of 25 micrograms every 3. Appelhof BC, et al. Triiodothyronine addition to paroxetine in I 8 hours until improvement occurs; the dosage may the treatment of major depressive disorder. J Clin Endocrinol then be reduced to 25 micrograms intravenously twice Metab 2004; 89: 6271–6. (liothyronine) daily. Obesity. Thyroid drugs have been tried in the treatment of obes- Liothyronine has also been given in the diagnosis of ity (p.2149) in euthyroid patients, but they produce only tempo- NOTE. The abbreviation T3 is often used for endogenous tri-io- hyperthyroidism in adults. Failure to suppress the up- rary weight loss, mainly of lean body-mass, and can produce se- dothyronine in medical and biochemical reports. Liotrix is USAN rious adverse effects, especially cardiac complications.1 for a mixture of liothyronine sodium with levothyroxine sodium. -

Potassium Hydroxide Cas N°: 1310-58-3
OECD SIDS POTASSIUM HYDROXIDE FOREWORD INTRODUCTION POTASSIUM HYDROXIDE CAS N°: 1310-58-3 UNEP PUBLICATIONS 1 OECD SIDS POTASSIUM HYDROXIDE SIDS Initial Assessment Report For SIAM 13 Bern, Switzerland, 6-9 November 2001 1. Chemical Name: Potassium hydroxide 2. CAS Number: 1310-58-3 3. Sponsor Country: Belgium Dr. Thaly LAKHANISKY J. Wytsman 16 B-1050 Brussels, Belgium Tel : + 32 2 642 5104 Fax : +32 2 642 5224 E-mail : [email protected] 4. Shared Partnership with: ICCA (Tessenderlo Chemie NV) 5. Roles/Responsibilities of the Partners: · Name of industry sponsor /consortium · Process used 6. Sponsorship History · How was the chemical or In 2001, ICCA (Tessenderlo Chemie NV)) had proposed sponsor category brought into the and prepared draft documents(Dossier, SIAR, SIAP). It was OECD HPV Chemicals submitted to the SIDS contact point of Belgium on May 2001. Programme? The draft documents were revised by Belgium after discussion with Tessenderlo Chemie NV. The revised draft was discussed in detail with Tessenderlo Chemie NV on June and July 2001. After agreement, the documents were finalized and the checklist was developed by jointly by Belgium and Tessenderlo Chemie NV 7. Review Process Prior to the SIAM: 8. Quality check process: 9. Date of Submission: 10. Date of last Update: February 2002 11. Comments: No testing 2 UNEP PUBLICATIONS OECD SIDS POTASSIUM HYDROXIDE SIDS INITIAL ASSESSMENT PROFILE CAS No. 1310-58-3 Chemical Name Potassium hydroxide Structural Formula KOH RECOMMENDATIONS The chemical is currently of low priority for further work. SUMMARY CONCLUSIONS OF THE SIAR Human Health Solid KOH is corrosive. -

Package Insert TECHNETIUM Tc99m GENERATOR for the Production of Sodium Pertechnetate Tc99m Injection Diagnostic Radiopharmaceuti
NDA 17693/S-025 Page 3 Package Insert TECHNETIUM Tc99m GENERATOR For the Production of Sodium Pertechnetate Tc99m Injection Diagnostic Radiopharmaceutical For intravenous use only Rx ONLY DESCRIPTION The technetium Tc99m generator is prepared with fission-produced molybdenum Mo99 adsorbed on alumina in a lead-shielded column and provides a means for obtaining sterile pyrogen-free solutions of sodium pertechnetate Tc99m injection in sodium chloride. The eluate should be crystal clear. With a pH of 4.5-7.5, hydrochloric acid and/or sodium hydroxide may have been used for Mo99 solution pH adjustment. Over the life of the generator, each elution will provide a yield of > 80% of the theoretical amount of technetium Tc99m available from the molybdenum Mo99 on the generator column. Each eluate of the generator should not contain more than 0.0056 MBq (0.15 µCi) of molybdenum Mo99 per 37 MBq, (1 mCi) of technetium Tc99m per administered dose at the time of administration, and not more than 10 µg of aluminum per mL of the generator eluate, both of which must be determined by the user before administration. Since the eluate does not contain an antimicrobial agent, it should not be used after twelve hours from the time of generator elution. PHYSICAL CHARACTERISTICS Technetium Tc99m decays by an isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging studies is listed in Table 1. Table 1. Principal Radiation Emission Data1 Radiation Mean %/Disintegration Mean Energy (keV) Gamma-2 89.07 140.5 1Kocher, David C., “Radioactive Decay Data Tables,” DOE/TIC-11026, p. -

Common Name: POTASSIUM CHLORATE HAZARD SUMMARY IDENTIFICATION REASON for CITATION HOW to DETERMINE IF YOU ARE BEING EXPOSED WORK
Common Name: POTASSIUM CHLORATE CAS Number: 3811-04-9 DOT Number: UN 1485 RTK Substance number: 1560 UN 2427 (Aqueous solution) Date: March 1998 Revision: October 2004 --------------------------------------------------------------------------- --------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Potassium Chlorate can affect you when breathed in. No occupational exposure limits have been established for * Contact can cause eye and skin irritation and burns. Potassium Chlorate. This does not mean that this substance * Breathing Potassium Chlorate can irritate the nose, throat is not harmful. Safe work practices should always be and lungs causing sneezing, coughing and sore throat. followed. * High levels can interfere with the ability of the blood to carry oxygen causing headache, weakness, dizziness and a WAYS OF REDUCING EXPOSURE blue color to the skin (methemoglobinemia). Higher levels * Where possible, enclose operations and use local exhaust can cause trouble breathing, collapse and even death. ventilation at the site of chemical release. If local exhaust * Repeated exposure may affect the kidneys and nervous ventilation or enclosure is not used, respirators should be system. worn. * Wear protective work clothing. IDENTIFICATION * Wash thoroughly immediately after exposure to Potassium Chlorate is a transparent, colorless crystal or Potassium Chlorate and at the end of the workshift. white powder. It is used as an oxidizing agent, and in * Post hazard and warning information in the work area. In explosives, matches, textile printing, disinfectants and addition, as part of an ongoing education and training bleaches. effort, communicate all information on the health and safety hazards of Potassium Chlorate to potentially REASON FOR CITATION exposed workers. * Potassium Chlorate is on the Hazardous Substance List because it is cited by DOT. -

Black Match” …………………………………………… P
Selected Pyrotechnic Publications of K.L. and B.J. Kosanke Part 5 (1998 through 2000) This book contains 134 pages Development of a Video Spectrometer …………………………………………… P. 435-445. Measurements of Glitter Flash Delay, Size and Duration ……………………… P. 446-449. Lift Charge Loss for a Shell to Remain in Mortar ……………………………… P. 450-450. Configuration and “Over-Load” Studies of Concussion Mortars ……………… P. 451-463. Quick Match – A Review and Study ……………………………………………… P. 464-479. Pyrotechnic Primes and Priming ………………………………………………… P. 480-495. Dud Shell Risk Assessment: NFPA Distances …………………………………… P. 496-499. Dud Shell Risk Assessment: Mortar Placement ………………………………… P. 500-503. Performance Study of Civil War Vintage Black Powder ……………………… P. 504-509. CAUTION: Very Fast “Black Match” …………………………………………… P. 510-512. Peak In-Mortar Aerial Shell Accelerations ……………………………………… P. 513-516. Firing Precision for Choreographed Displays …………………………………… P. 517-518. Sticky Match and Quick Match: Temperature Dependent Burn Times ……… P. 519-523. Mortar Separations in Troughs and Drums …………………………………… P. 524-530. Preliminary Study of the Effect of Ignition Stimulus on Aerial Shell Lift Performance …………………………………………………… P. 531-535. Pyrotechnic Particle Morphologies – Metal Fuels ……………………………… P. 536-542. Peak Mortar Pressures When Firing Spherical Aerial Shells …………………… P. 543-544. Indoor Pyrotechnic Electrostatic Discharge Hazard …………………………… P. 545-545. Pyrotechnic Particle Morphology – Low Melting Point Oxidizers ……………… P. 546-556. An earlier version -

Toasting a Gummy Candy
WARNING NOTICE The experiments described in these materials are potentially hazardous. Among other things, the experiments should include the following safety measures: a high level of safety training, special facilities and equipment, the use of proper personal protective equipment, and supervision by appropriate individuals. You bear the sole responsibility, liability, and risk for the implementation of such safety procedures and measures. MIT and Dow shall have no responsibility, liability, or risk for the content or implementation of any of the material presented. Legal Notice TOASTING A GUMMY CANDY A photo taken at MIT 150th Celebration Open House Under the Dome April 30, 2011. Courtesy of Nathan Sanders. Used with permission. Abstract A gummy bear candy is oxidized using KClO3 as the oxidizing agent in a dramatic reaction, which releases a large amount of energy and results in the formation of harmless products KCl, CO2, and H2O. Materials Gummy Bear Candy 25mm Heavy-Walled Borosilicate Test Tube Potassium chlorate, KClO3 Cast Iron Support Stand for Test Tube Clamp Spatula Test Tube Clamp Explosion Shield Portable Butane Burner or Lab Burner Long laboratory tweezers Face shield Safety Potassium chlorate is a strong oxidizer, which could ignite if placed into contact with other materials. It is a known skin, eye, and respiratory tract irritant. Potassium chlorate should always be fresh and weighed on a balance right before using it. It’s a good idea to mass it directly into the test tube you are going to use in this experiment and cover the opening with paraffin wax paper to protect the contents from any contamination. -

M-80 (Explosive) - Wikipedia,Visited the Free Encyclopediaon 8/14/2015 Page 1 of 4
M-80 (explosive) - Wikipedia,visited the free encyclopediaon 8/14/2015 Page 1 of 4 M-80 (explosive) From Wikipedia, the free encyclopedia For other uses, see M80. M-80s are an American class of large firecrackers, sometimes called SALUTES.[1] The Simulator, Artillery, M-80 was originally made in the early 20th century by the U.S. military to simulate explosives or artillery fire;[2] later, M-80s were manufactured as fireworks. Traditionally, M-80s were made from a small cardboard 1 tube, often red, approximately 1 ⁄2 inches (3.8 cm) long 9 and ⁄16 inch (1.4 cm) inside diameter, with a fuse or wick coming out of the side; this type fuse is commonly known as cannon fuse or Visco fuse, after a company responsible for standardizing the product. The tubes M-80 Salute (BOOTLEG) often hold approximately 2½–3 grams of pyrotechnic flash powder; many sources state that an M-80 carries 3 grams of powder.[3] Contents ◾ 1 Legality ◾ 1.1 Canada ◾ 1.2 United States ◾ 2 Accidents ◾ 3 References ◾ 4 Further reading Legality Canada https://en.wikipedia.org/wiki/M-80_(explosive) 8/14/2015 M-80 (explosive) - Wikipedia,visited the free encyclopediaon 8/14/2015 Page 2 of 4 M-80s are not authorized under the law, thus making importation, possession, transportation, storage or manufacturing illegal in Canada.[4] Firecrackers, including the M-80, can be purchased from Native Reserves in Canada, as they have different governing laws. United States Due to property damages and bodily harm caused by M-80s, Class C fireworks—now known as consumer fireworks (class 1.4G), as opposed to display fireworks (which were Class B, and are now 1.3G)[5]— civilians require a license, issued by federal authorities, for pyrotechnic devices containing a charge in excess of 50 milligrams of pyrotechnic flash powder. -
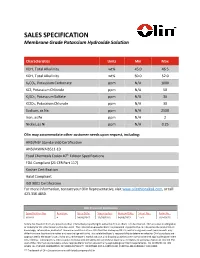
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution Characteristics Units Min Max KOH, Total Alkalinity wt% 45.0 46.5 KOH, Total Alkalinity wt% 50.0 52.0 K2CO3, Potassium Carbonate ppm N/A 1000 KCl, Potassium Chloride ppm N/A 50 K2SO4, Potassium Sulfate ppm N/A 30 KClO3, Potassium Chlorate ppm N/A 30 Sodium, as Na ppm N/A 2500 Iron, as Fe ppm N/A 2 Nickel, as Ni ppm N/A 0.25 Olin may accommodate other customer needs upon request, including: ANSI/NSF Standard 60 Certification ANSI/AWWA B511-10 Food Chemicals Codex 10th Edition Specifications FDA Compliant (21 CFR Part 117) Kosher Certification Halal Compliant ISO 9001 Certification For more information, contact your Olin Representative, visit www.olinchloralkali.com, or call 423.336.4850. Olin Document Information Specification No: Revision: Issue Date: Supersedes: Review Date: Sheet No.: Form No.: KOH-S1 4 04/18/2017 12/28/2016 04/18/2022 1 of 1 102-00526 Notice: No freedom from any patent or other intellectual property rights owned by Olin or others is to be inferred. Olin assumes no obligation or liability for the information in this document. The information provided herein is presented in good faith a nd is based on the best of Olin’s knowledge, information, and belief. Since use conditions at non-Olin facilities are beyond Olin’s control and government requirements may differ from one location to another and may change with time, it is solely the Buyer’s responsibility to determine whether Olin’s products are appropriate for the Buyer’s use, and to assure the Buyer’s workplace, use, and disposal practices are in compliance with appl icable government requirements. -

2013 Fireworks Safety Standards Development Status Report
FIREWORKS SAFETY STANDARDS DEVELOPMENT PROJECT FY 2013 STATUS REPORT October 2013 For further information contact: Dr. Christopher J. Musto, Project Manager Directorate for Laboratory Sciences U.S. Consumer Product Safety Commission (301) 987-2090 [email protected] Dr. Andrew Lock Directorate for Laboratory Sciences U.S. Consumer Product Safety Commission (301) 987-2099 [email protected] This report was prepared by the CPSC staff, has not been reviewed or approved by, and may not necessarily reflect the views of, the Commission. EXECUTIVE SUMMARY The U.S Consumer Product Safety Commission (CPSC, the Commission) is evaluating the need to amend its regulations for fireworks devices. CPSC staff evaluated issues concerning the explosive power of aerial fireworks. Aerial fireworks devices intended to produce an audible effect are limited to 130 mg (2 grains) of pyrotechnic composition, according to 16 C.F.R. § 1500.17(a)(3). Because the regulation only limits the amount of pyrotechnic composition for devices intended to produce an audible effect, compliance determinations can be made only after a subjective determination of intent to produce an audible effect. In 2012, staff conducted testing to evaluate two different approaches to replacing the regulation limiting pyrotechnic composition of devices intended to produce an audible effect; staff considered potential alternative regulations, based on the explosive power of fireworks devices. The American Fireworks Standards Laboratory (AFSL) had previously developed a “Black Powder Equivalency Test.” CPSC staff investigated and found that this test can distinguish between the energetics of black powder, hybrid powders, and flash powder, but the test does not consider the particular construction of a shell. -

Washington State Patrol Crime Laboratory Division Materials
Washington State Patrol Crime Laboratory Division Materials Analysis EXPLOSIVES TRAINING MANUAL May 2018 Washington State Patrol Crime Laboratory Division Explosives Laboratory Training Manual Contents 1 INTRODUCTION ..................................................................................................................................... 7 1.1 PURPOSE AND SCOPE ................................................................................................................... 7 1.2 ORGANIZATION OF THE TRAINING MANUAL ................................................................................ 7 2 EXPLOSIVES ANALYSIS OVERVIEW ........................................................................................................ 9 2.1 OBJECTIVES ................................................................................................................................... 9 2.2 OVERVIEW ..................................................................................................................................... 9 2.2.1 Definitions ............................................................................................................................. 9 2.3 SUGGESTED READINGS ............................................................................................................... 11 2.3.1 Introduction ........................................................................................................................ 11 2.3.2 Scene ..................................................................................................................................