Potassium Hydroxide Cas N°: 1310-58-3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Name: POTASSIUM CHLORATE HAZARD SUMMARY IDENTIFICATION REASON for CITATION HOW to DETERMINE IF YOU ARE BEING EXPOSED WORK
Common Name: POTASSIUM CHLORATE CAS Number: 3811-04-9 DOT Number: UN 1485 RTK Substance number: 1560 UN 2427 (Aqueous solution) Date: March 1998 Revision: October 2004 --------------------------------------------------------------------------- --------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Potassium Chlorate can affect you when breathed in. No occupational exposure limits have been established for * Contact can cause eye and skin irritation and burns. Potassium Chlorate. This does not mean that this substance * Breathing Potassium Chlorate can irritate the nose, throat is not harmful. Safe work practices should always be and lungs causing sneezing, coughing and sore throat. followed. * High levels can interfere with the ability of the blood to carry oxygen causing headache, weakness, dizziness and a WAYS OF REDUCING EXPOSURE blue color to the skin (methemoglobinemia). Higher levels * Where possible, enclose operations and use local exhaust can cause trouble breathing, collapse and even death. ventilation at the site of chemical release. If local exhaust * Repeated exposure may affect the kidneys and nervous ventilation or enclosure is not used, respirators should be system. worn. * Wear protective work clothing. IDENTIFICATION * Wash thoroughly immediately after exposure to Potassium Chlorate is a transparent, colorless crystal or Potassium Chlorate and at the end of the workshift. white powder. It is used as an oxidizing agent, and in * Post hazard and warning information in the work area. In explosives, matches, textile printing, disinfectants and addition, as part of an ongoing education and training bleaches. effort, communicate all information on the health and safety hazards of Potassium Chlorate to potentially REASON FOR CITATION exposed workers. * Potassium Chlorate is on the Hazardous Substance List because it is cited by DOT. -

Exposure to Potassium Hydroxide Can Cause Headache, Eye Contact Dizziness, Nausea and Vomiting
Right to Know Hazardous Substance Fact Sheet Common Name: POTASSIUM HYDROXIDE Synonyms: Caustic Potash; Lye; Potassium Hydrate CAS Number: 1310-58-3 Chemical Name: Potassium Hydroxide (KOH) RTK Substance Number: 1571 Date: May 2001 Revision: January 2010 DOT Number: UN 1813 Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Potassium Hydroxide is an odorless, white or slightly yellow, Hazard Summary flakey or lumpy solid which is often in a water solution. It is Hazard Rating NJDOH NFPA used in making soap, as an electrolyte in alkaline batteries and HEALTH - 3 in electroplating, lithography, and paint and varnish removers. FLAMMABILITY - 0 Liquid drain cleaners contain 25 to 36% of Potassium REACTIVITY - 1 Hydroxide. CORROSIVE POISONOUS GASES ARE PRODUCED IN FIRE DOES NOT BURN Reasons for Citation Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; f Potassium Hydroxide is on the Right to Know Hazardous 4=severe Substance List because it is cited by ACGIH, DOT, NIOSH, NFPA and EPA. f Potassium Hydroxide can affect you when inhaled and by f This chemical is on the Special Health Hazard Substance passing through the skin. List. f Potassium Hydroxide is a HIGHLY CORROSIVE CHEMICAL and contact can severely irritate and burn the skin and eyes leading to eye damage. f Contact can irritate the nose and throat. f Inhaling Potassium Hydroxide can irritate the lungs. SEE GLOSSARY ON PAGE 5. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. FIRST AID f Exposure to Potassium Hydroxide can cause headache, Eye Contact dizziness, nausea and vomiting. -

PCTM 17 ISSUED-1996 Method to Determine Rosin Acids in Tall Oil
PCTM 17 ISSUED-1996 Method to determine rosin acids in tall oil Scope 3. Methyl sulfuric acid solution, 20% - Caution: This method covers the determination of rosin acids in Slowly pour 100 g of concentrated sulfuric acid (- tall oils containing more than 15% rosin acids. 96%), while stirring constantly, into 400 g of methanol. This method may not be applicable to adducts or 4 . Thymol blue indicator - Weigh 0.1 g thymol blue in derivatives of tall oils, or other naval stores products. 100 mL methanol. Fatty acids are esterified by methanol in the presence of sulfuric Sample Preparation acid catalyst, and rosin acids are determined by titration after neutralization of the sulfuric acid. 1. Dissolve 5 ± 0.5 g of sample, weighed to the nearest 0.001 g, into a 250-m1. Erlenmeyer flask. Apparatus 2. Add 100 mL of methanol and swirl to dissolve. 3. Add 5.0 mL of methyl sulfuric acid solution. 1. Beaker, tall-form, 300-mL capacity. 4. Connect the flask to the condenser and reflux for 2. Buret, 50-mL capacity with 0.1-mL divisions. 30 minutes. Allow the flask to cool to Electronic burets are preferable for increased approximately room temperature. accuracy and precision. 3. Erlenmeyer flask, 250-mL flat-bottom fitted with a Method A - Potentiometric Titration condenser. 4. pH meter, capable of reading ± 0.1 pH over a 1. Titrate with the KOH solution to a fixed pH of range of pH 1 to pH 13 in alcoholic "solutions. 4.0, the first end point. 5. Pipet, 5-mL. -

SAFETY DATA SHEET Potassium Hydroxide, Liquid 45-50% Revision Date: 2020-06-15 Version: 1.0
SAFETY DATA SHEET Potassium Hydroxide, Liquid 45-50% Revision date: 2020-06-15 Version: 1.0 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name: Potassium Hydroxide, Liquid 45-50% Synonyms: Chemical manufacturing, fertilizer, batteries, soaps Product Form: Liquid Recommended use of the chemical and restrictions on use Recommended Use: Professional use, Industrial use. Chemical manufacturing, fertilizer, batteries, soaps Restrictions on Use: Use as recommended by the label Details of the supplier and of the safety data sheet Supplier Tersus Environmental, LLC 1116 Colonial Club Rd Wake Forest, NC 27587 Phone: +1-919-453-5577 Email: [email protected] Contact Person David F. Alden Phone: +1-919-453-5577 x2002 Email: [email protected] Emergency telephone number For leak, fire, spill or accident emergencies, call: +1-919-453-5577 (Tersus Office Hours, 8:00 AM to 5:00 PM Eastern) +1-800-424-9300 (Chemtrec 24 Hour Service – Emergency Only) +1-703-527-3887 (Chemtrec Outside United States 24 Hour Service – Emergency Only) +1-919-638-7892 Gary M. Birk (Outside office hours) 2. HAZARD IDENTIFICATION Relevant identified uses of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) GHS label elements, including precautionary statements: Signal Word: Danger Pictogram(s): GHS05 GHS07 Page 1 of 10 Potassium Hydroxide, Liquid 45-50% Revision date: 2020-06-15 Version: 1.0 Hazard statement H290 May be corrosive to metals. H302 Harmful if swallowed. H314 Causes severe skin burns and eye damage H318 Causes serious eye damage. H402 Harmful to aquatic life. Precautionary statement P234 Keep only in original container. -

Potassium Hydroxide Safety Data Sheet According to Federal Register / Vol
Potassium Hydroxide Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 10/09/2004 Revision date: 02/06/2018 Supersedes: 02/06/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Potassium Hydroxide CAS-No. : 1310-58-3 Product code : LC19190 Formula : KOH Synonyms : caustic potash / caustic potash dry / caustic potash, dry solid, flake, bead or granular / caustic potash, solid / caustic potash,solid / hydrate of potash / hydrate of potassium / hydroxide of potash / hydroxide of potassium / lye (=potassium hydroxide) / potash / potash hydrate / potash lye / potassium hydrate / potassium hydroxide (K(OH)) / potassium hydroxide dry / potassium hydroxide pellets / potassium hydroxide, dry solid, flake, bead or granular / potassium hydroxide, electrolytical, solid / potassium hydroxide, solid / Potassium hydroxide, solid / potassium lye 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Acute toxicity (oral) H302 Harmful if swallowed Category 4 Skin corrosion/irritation H314 Causes severe skin burns and eye damage Category 1A Hazardous to the aquatic H402 Harmful to aquatic life environment - Acute Hazard Category 3 Full text of H statements : see section 16 2.2. -

Toasting a Gummy Candy
WARNING NOTICE The experiments described in these materials are potentially hazardous. Among other things, the experiments should include the following safety measures: a high level of safety training, special facilities and equipment, the use of proper personal protective equipment, and supervision by appropriate individuals. You bear the sole responsibility, liability, and risk for the implementation of such safety procedures and measures. MIT and Dow shall have no responsibility, liability, or risk for the content or implementation of any of the material presented. Legal Notice TOASTING A GUMMY CANDY A photo taken at MIT 150th Celebration Open House Under the Dome April 30, 2011. Courtesy of Nathan Sanders. Used with permission. Abstract A gummy bear candy is oxidized using KClO3 as the oxidizing agent in a dramatic reaction, which releases a large amount of energy and results in the formation of harmless products KCl, CO2, and H2O. Materials Gummy Bear Candy 25mm Heavy-Walled Borosilicate Test Tube Potassium chlorate, KClO3 Cast Iron Support Stand for Test Tube Clamp Spatula Test Tube Clamp Explosion Shield Portable Butane Burner or Lab Burner Long laboratory tweezers Face shield Safety Potassium chlorate is a strong oxidizer, which could ignite if placed into contact with other materials. It is a known skin, eye, and respiratory tract irritant. Potassium chlorate should always be fresh and weighed on a balance right before using it. It’s a good idea to mass it directly into the test tube you are going to use in this experiment and cover the opening with paraffin wax paper to protect the contents from any contamination. -

Potassium Hydroxide, 0.1N (0.1M) in Ethanol Safety Data Sheet According to Federal Register / Vol
Potassium Hydroxide, 0.1N (0.1M) in Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/19/2013 Revision date: 02/06/2018 Supersedes: 02/06/2018 Version: 1.3 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Potassium Hydroxide, 0.1N (0.1M) in Ethanol Product code : LC19310 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids H225 Highly flammable liquid and vapour Category 2 Serious eye damage/eye H319 Causes serious eye irritation irritation Category 2A Carcinogenicity Category H350 May cause cancer 1A Reproductive toxicity H361 Developmental toxicity (oral) Category 2 Specific target organ H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) toxicity (single exposure) Category 1 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-US labeling Hazard pictograms (GHS-US) : GHS02 GHS07 GHS08 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H225 - Highly flammable liquid and vapour H319 - Causes serious eye irritation H350 - May cause cancer H361 - Developmental toxicity (oral) H370 - Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Precautionary statements (GHS-US) : P201 - Obtain special instructions before use. -

Potassium Hydroxide (P6310)
Potassium hydroxide ACS Reagent Product Number P 6310 Store at Room Temperature 22,147-3 is an exact replacement for P 6310 Product Description Preparation Instructions Molecular Formula: KOH This product is soluble in water (100 mg/ml), yielding a Molecular Weight: 56.11 clear, colorless solution. Potassium hydroxide is also CAS Number: 1310-58-3 soluble in alcohol (1 part in 3) and glycerol (1 part Melting point: 360 °C, 380 °C (anhydrous)1 in 2.5). The dissolution of potassium hydroxide in water or alcohol is a highly exothermic (heat- This product is in the form of pellets. It is designated producing) process.1 as ACS Reagent grade, and meets the specifications of the American Chemical Society (ACS) for reagent Storage/Stability chemicals. Potassium hydroxide rapidly absorbs carbon dioxide and water from the air and deliquesces.1 Potassium Potassium hydroxide (KOH) is a caustic reagent that is hydroxide solutions should be stored in plastic bottles widely used to neutralize acids and prepare potassium (polyethylene or polypropylene). KOH solutions will salts of reagents. It is used in a variety of large-scale etch glass over a period of just a few days. applications, such as the manufacture of soap, the mercerizing of cotton, electroplating, photoengraving, References and lithography.1 1. The Merck Index, 12th ed., Entry# 7806. 2. Philip, N. S., and Green, D. M., Recovery and Potassium hydroxide is used in the analysis of bone enhancement of faded cleared and double stained and cartilage samples by histology.2,3 A protocol for specimens. Biotech. Histochem., 75(4), 193-196 the amplification of DNA from single cells by PCR that (2000). -
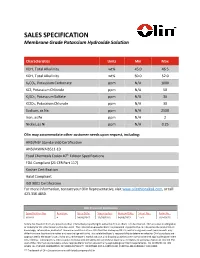
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution Characteristics Units Min Max KOH, Total Alkalinity wt% 45.0 46.5 KOH, Total Alkalinity wt% 50.0 52.0 K2CO3, Potassium Carbonate ppm N/A 1000 KCl, Potassium Chloride ppm N/A 50 K2SO4, Potassium Sulfate ppm N/A 30 KClO3, Potassium Chlorate ppm N/A 30 Sodium, as Na ppm N/A 2500 Iron, as Fe ppm N/A 2 Nickel, as Ni ppm N/A 0.25 Olin may accommodate other customer needs upon request, including: ANSI/NSF Standard 60 Certification ANSI/AWWA B511-10 Food Chemicals Codex 10th Edition Specifications FDA Compliant (21 CFR Part 117) Kosher Certification Halal Compliant ISO 9001 Certification For more information, contact your Olin Representative, visit www.olinchloralkali.com, or call 423.336.4850. Olin Document Information Specification No: Revision: Issue Date: Supersedes: Review Date: Sheet No.: Form No.: KOH-S1 4 04/18/2017 12/28/2016 04/18/2022 1 of 1 102-00526 Notice: No freedom from any patent or other intellectual property rights owned by Olin or others is to be inferred. Olin assumes no obligation or liability for the information in this document. The information provided herein is presented in good faith a nd is based on the best of Olin’s knowledge, information, and belief. Since use conditions at non-Olin facilities are beyond Olin’s control and government requirements may differ from one location to another and may change with time, it is solely the Buyer’s responsibility to determine whether Olin’s products are appropriate for the Buyer’s use, and to assure the Buyer’s workplace, use, and disposal practices are in compliance with appl icable government requirements. -

CHANGES in Ph ASSOCIATED with the APPLICATION of AMMONIA and POTASSIUM HYDROXIDE to DIAPAUSING EGGS of TELEOGRYLLUS OOMMODUS (WALK.) (ORTHOPTERA: GRYLLIDAE)
CHANGES IN pH ASSOCIATED WITH THE APPLICATION OF AMMONIA AND POTASSIUM HYDROXIDE TO DIAPAUSING EGGS OF TELEOGRYLLUS OOMMODUS (WALK.) (ORTHOPTERA: GRYLLIDAE) By T. W. HOGAN* [Manuscript received September 9, 1964] Summary Diapausing eggs of T. commodus were exposed to a:mmonia evolved from solutions of ammonium hydroxide in desiccators for specific periods. It was found that the pH of the egg contents increased with increase in concentration of the ammonium hydroxide and the period of exposure. Toxic symptoms and mortality of eggs were associated with a rise of pH exceeding 0·4 of a unit. This occurred at the higher concentrations, viz. exposure for 3 days to O· 3M or for 2-3 days to 0 ·IM ammonium hydroxide. The most favourable effect on the termination of diapause was with exposure for 3 days to O· 01M ammonium hydroxide, which raised the pH to 7· O. This was also the highest non-toxic concentration. On the basis that the rise in pH might be the causal factor for the termination of diapause, the effect of potassium hydroxide solutions on the pH of the eggs was tested and found to be moderately effective both in this regard and in accelerating the rate of termination of diapause. However, there was some termination of diapause before any significant rise in pH occurred. It appears, therefore, that the action of ammonia and of potassium hydroxide must have a common factor other than raising the pH of the egg contents. 1. INTRODUCTION It has been shown that when diapausing eggs of Teleogryllu8 commodu8 (Walk.) are exposed to ammonia evolved from solutions of ammonium hydroxide for an adequate period, diapause is terminated in a high proportion of the eggs. -

Study of the Reactions of Chloroform with Carbonyl Compounds
A STUDY OF .ACTIONS OF CHLOROFORM WITH CARBOHTL COMPOUNDS by ERNEST ALVA IKENBERRY A. B., McPherson College, 1947 A THESIS Submitted In partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Chemistry KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE 1951 mervV LO II TABLE OF CONTENTS ia INTRODUCTION 1 LITER. :,\IU! 2 DISCOS 3 10» 6 Products from Potassium Hydroxide 8atalyzed Pveactions . 8 Products from Quaternary Base Catalyzed Reactions ........ 13 Polymer-plastics Obtained from Chloroform- crotonaldehyde Reactions 19 Attempted Free-radical Type Reactions of Chloroform and Cinnamaldehyde 20 EXP. TAL 21 Prepare tion of Starting Materials 21 The Reaction of Chloroform and Croton- aldehyde in the Pressence of Solid Potassium Hydroxide 22 The Reaction of Chloroform and Cinnam- aldehyde and Mesityl Oxide in the Presence of Solid Potassium Hydroxide . 24 The Reaction of Chloroform and Croton- aldehyde in the Presence of an Organic Quaternary Base 25 Attempted Free-radical Type Reactions of Chloroform and Cinnamaldehyde 27 Number of Experiments Carried Oub 28 SUMMARY. 29 irinrnwTifiniwiB 31 LITERATURE CITED - 2 — INTRODUCTION Since Willgerodt (1) in 1881 reacted chloroform with acetone in the presence of solid potassium hydroxide to form trichloromethyl dimethyl carbinol, this reaction has been extended to a great number of aldehydes and ketones. G R» 0H Rt __ __ R—0=0 + R-CC13 * R C 0H cci3 The mechanism is thought to follow the usual course of base catalyzed condensations in which addition occurs between the polarized carbonyl group and the base generated trichloro- methyl anion. This investigation was undertaken to study the reaction of chloroform with acrylaldehydes. -
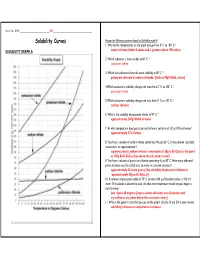
Solubility Curves Answer the Following Questions Based on Solubility Graph a 1
Name, Date, Hr/Per_______________________________ KEY ________________________________________ Solubility Curves Answer the following questions based on Solubility graph A 1. Why do the temperatures on the graph only go from 0º C to 100º C ? SOLUBILITY GRAPH A water is frozen below 0 celcus and is gaseous above 100 celcius 2. Which substance is most soluble at 60º C ? potassium iodide 3. Which two substances have the same solubility at 80º C ? potassium chlorate & sodium chloride [both at 40g/100mL water] 4.Which substance’s solubility changes the most from 0º C to 100º C ? potassium nitrate 5.Which substance’s solubility changes the least from 0º C to 100º C ? sodium chloride 6. What is the solubility of potassium nitrate at 90º C ? approximately 200g/100mL of water 7. At what temperature does potassium iodide have a solubility of 150 g/ 100 cm3 water? approximately 22.5 Celcius 8. You have a solution of sodium nitrate containing 140 g at 65º C. Is the solution saturated, unsaturated, or supersaturated ? supersaturated [sodium nitrate is saturated at 130g at 65 Celcius; the point at 140g & 65 Celcius lies above the saturation curve] 9. You have a solution of potassium chlorate containing 4 g at 65º C. How many additional grams of solute must be added to it, to make the solution saturated ? approximately 26 more grams [the solubility of potassium chlorate is `approximately 30g at 65 Celcius] 10. A solution of potassium iodide at 70º C contains 200 g of dissolved solute in 100 cm 3 water. The solution is allowed to cool.