The Hungry Dragon Combustion Reactions SCIENTIFIC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potassium Hydroxide Cas N°: 1310-58-3
OECD SIDS POTASSIUM HYDROXIDE FOREWORD INTRODUCTION POTASSIUM HYDROXIDE CAS N°: 1310-58-3 UNEP PUBLICATIONS 1 OECD SIDS POTASSIUM HYDROXIDE SIDS Initial Assessment Report For SIAM 13 Bern, Switzerland, 6-9 November 2001 1. Chemical Name: Potassium hydroxide 2. CAS Number: 1310-58-3 3. Sponsor Country: Belgium Dr. Thaly LAKHANISKY J. Wytsman 16 B-1050 Brussels, Belgium Tel : + 32 2 642 5104 Fax : +32 2 642 5224 E-mail : [email protected] 4. Shared Partnership with: ICCA (Tessenderlo Chemie NV) 5. Roles/Responsibilities of the Partners: · Name of industry sponsor /consortium · Process used 6. Sponsorship History · How was the chemical or In 2001, ICCA (Tessenderlo Chemie NV)) had proposed sponsor category brought into the and prepared draft documents(Dossier, SIAR, SIAP). It was OECD HPV Chemicals submitted to the SIDS contact point of Belgium on May 2001. Programme? The draft documents were revised by Belgium after discussion with Tessenderlo Chemie NV. The revised draft was discussed in detail with Tessenderlo Chemie NV on June and July 2001. After agreement, the documents were finalized and the checklist was developed by jointly by Belgium and Tessenderlo Chemie NV 7. Review Process Prior to the SIAM: 8. Quality check process: 9. Date of Submission: 10. Date of last Update: February 2002 11. Comments: No testing 2 UNEP PUBLICATIONS OECD SIDS POTASSIUM HYDROXIDE SIDS INITIAL ASSESSMENT PROFILE CAS No. 1310-58-3 Chemical Name Potassium hydroxide Structural Formula KOH RECOMMENDATIONS The chemical is currently of low priority for further work. SUMMARY CONCLUSIONS OF THE SIAR Human Health Solid KOH is corrosive. -

Common Name: POTASSIUM CHLORATE HAZARD SUMMARY IDENTIFICATION REASON for CITATION HOW to DETERMINE IF YOU ARE BEING EXPOSED WORK
Common Name: POTASSIUM CHLORATE CAS Number: 3811-04-9 DOT Number: UN 1485 RTK Substance number: 1560 UN 2427 (Aqueous solution) Date: March 1998 Revision: October 2004 --------------------------------------------------------------------------- --------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Potassium Chlorate can affect you when breathed in. No occupational exposure limits have been established for * Contact can cause eye and skin irritation and burns. Potassium Chlorate. This does not mean that this substance * Breathing Potassium Chlorate can irritate the nose, throat is not harmful. Safe work practices should always be and lungs causing sneezing, coughing and sore throat. followed. * High levels can interfere with the ability of the blood to carry oxygen causing headache, weakness, dizziness and a WAYS OF REDUCING EXPOSURE blue color to the skin (methemoglobinemia). Higher levels * Where possible, enclose operations and use local exhaust can cause trouble breathing, collapse and even death. ventilation at the site of chemical release. If local exhaust * Repeated exposure may affect the kidneys and nervous ventilation or enclosure is not used, respirators should be system. worn. * Wear protective work clothing. IDENTIFICATION * Wash thoroughly immediately after exposure to Potassium Chlorate is a transparent, colorless crystal or Potassium Chlorate and at the end of the workshift. white powder. It is used as an oxidizing agent, and in * Post hazard and warning information in the work area. In explosives, matches, textile printing, disinfectants and addition, as part of an ongoing education and training bleaches. effort, communicate all information on the health and safety hazards of Potassium Chlorate to potentially REASON FOR CITATION exposed workers. * Potassium Chlorate is on the Hazardous Substance List because it is cited by DOT. -

Toasting a Gummy Candy
WARNING NOTICE The experiments described in these materials are potentially hazardous. Among other things, the experiments should include the following safety measures: a high level of safety training, special facilities and equipment, the use of proper personal protective equipment, and supervision by appropriate individuals. You bear the sole responsibility, liability, and risk for the implementation of such safety procedures and measures. MIT and Dow shall have no responsibility, liability, or risk for the content or implementation of any of the material presented. Legal Notice TOASTING A GUMMY CANDY A photo taken at MIT 150th Celebration Open House Under the Dome April 30, 2011. Courtesy of Nathan Sanders. Used with permission. Abstract A gummy bear candy is oxidized using KClO3 as the oxidizing agent in a dramatic reaction, which releases a large amount of energy and results in the formation of harmless products KCl, CO2, and H2O. Materials Gummy Bear Candy 25mm Heavy-Walled Borosilicate Test Tube Potassium chlorate, KClO3 Cast Iron Support Stand for Test Tube Clamp Spatula Test Tube Clamp Explosion Shield Portable Butane Burner or Lab Burner Long laboratory tweezers Face shield Safety Potassium chlorate is a strong oxidizer, which could ignite if placed into contact with other materials. It is a known skin, eye, and respiratory tract irritant. Potassium chlorate should always be fresh and weighed on a balance right before using it. It’s a good idea to mass it directly into the test tube you are going to use in this experiment and cover the opening with paraffin wax paper to protect the contents from any contamination. -
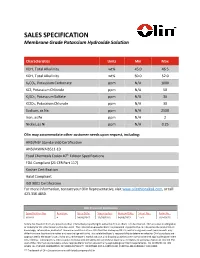
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution Characteristics Units Min Max KOH, Total Alkalinity wt% 45.0 46.5 KOH, Total Alkalinity wt% 50.0 52.0 K2CO3, Potassium Carbonate ppm N/A 1000 KCl, Potassium Chloride ppm N/A 50 K2SO4, Potassium Sulfate ppm N/A 30 KClO3, Potassium Chlorate ppm N/A 30 Sodium, as Na ppm N/A 2500 Iron, as Fe ppm N/A 2 Nickel, as Ni ppm N/A 0.25 Olin may accommodate other customer needs upon request, including: ANSI/NSF Standard 60 Certification ANSI/AWWA B511-10 Food Chemicals Codex 10th Edition Specifications FDA Compliant (21 CFR Part 117) Kosher Certification Halal Compliant ISO 9001 Certification For more information, contact your Olin Representative, visit www.olinchloralkali.com, or call 423.336.4850. Olin Document Information Specification No: Revision: Issue Date: Supersedes: Review Date: Sheet No.: Form No.: KOH-S1 4 04/18/2017 12/28/2016 04/18/2022 1 of 1 102-00526 Notice: No freedom from any patent or other intellectual property rights owned by Olin or others is to be inferred. Olin assumes no obligation or liability for the information in this document. The information provided herein is presented in good faith a nd is based on the best of Olin’s knowledge, information, and belief. Since use conditions at non-Olin facilities are beyond Olin’s control and government requirements may differ from one location to another and may change with time, it is solely the Buyer’s responsibility to determine whether Olin’s products are appropriate for the Buyer’s use, and to assure the Buyer’s workplace, use, and disposal practices are in compliance with appl icable government requirements. -
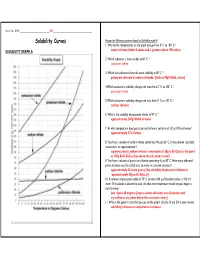
Solubility Curves Answer the Following Questions Based on Solubility Graph a 1
Name, Date, Hr/Per_______________________________ KEY ________________________________________ Solubility Curves Answer the following questions based on Solubility graph A 1. Why do the temperatures on the graph only go from 0º C to 100º C ? SOLUBILITY GRAPH A water is frozen below 0 celcus and is gaseous above 100 celcius 2. Which substance is most soluble at 60º C ? potassium iodide 3. Which two substances have the same solubility at 80º C ? potassium chlorate & sodium chloride [both at 40g/100mL water] 4.Which substance’s solubility changes the most from 0º C to 100º C ? potassium nitrate 5.Which substance’s solubility changes the least from 0º C to 100º C ? sodium chloride 6. What is the solubility of potassium nitrate at 90º C ? approximately 200g/100mL of water 7. At what temperature does potassium iodide have a solubility of 150 g/ 100 cm3 water? approximately 22.5 Celcius 8. You have a solution of sodium nitrate containing 140 g at 65º C. Is the solution saturated, unsaturated, or supersaturated ? supersaturated [sodium nitrate is saturated at 130g at 65 Celcius; the point at 140g & 65 Celcius lies above the saturation curve] 9. You have a solution of potassium chlorate containing 4 g at 65º C. How many additional grams of solute must be added to it, to make the solution saturated ? approximately 26 more grams [the solubility of potassium chlorate is `approximately 30g at 65 Celcius] 10. A solution of potassium iodide at 70º C contains 200 g of dissolved solute in 100 cm 3 water. The solution is allowed to cool. -

Environmental Health & Safety
Environmental Health & Safety Chemical Safety Program Chemical Segregation & Incompatibilities Guidelines Class of Recommended Incompatible Possible Reaction Examples Chemical Storage Method Materials If Mixed Corrosive Acids Mineral Acids – Separate cabinet or storage area Flammable Liquids Heat Chromic Acid away from potential water Flammable Solids Hydrogen Chloride sources, i.e. under sink Bases Hydrochloric Acid Oxidizers Gas Generation Nitric Acid Poisons Perchloric Acid Violent Phosphoric Acid Reaction Sulfuric Acid Corrosive Bases/ Ammonium Hydroxide Separate cabinet or storage area Flammable Liquids Heat Caustics Sodium Hydroxide away from potential water Flammable Solids Sodium Bicarbonate sources, i.e. under sink Acids Gas Generation Oxidizers Poisons Violent Reaction Explosives Ammonium Nitrate Secure location away from Flammable Liquids Nitro Urea other chemicals Oxidizers Picric Acid Poisons Explosion Hazard Trinitroaniline Acids Trinitrobenzene Bases Trinitrobenzoic Acid Trinitrotoluene Urea Nitrate Flammable Liquids Acetone Grounded flammable storage Acids Fire Hazard Benzene cabinet of flammable storage Bases Diethyl Ether refrigerator Oxidizers Methanol Poisons Heat Ethanol Toluene Violent Glacial Acetic Acid Reaction Flammable Solids Phosphorus Separate dry cool area Acids Fire Hazard Magnesium Bases Heat Oxidizers Violent Poisons Reaction Sodium Hypochlorite Spill tray that is separate from Reducing Agents Fire Oxidizers Benzoyl Peroxide flammable and combustible Flammables Hazard Potassium Permanganate materials Combustibles -

UNITED STATES of AMERICA, Plaintiff, 0.6SEP 14, PH 3:I5
UNITED STATES DISTRICT COURT 0.6SEP 14, PH 3:i5 FOR THE DISTRICT OF NEW MEXICO UNITED STATES OF AMERICA, ) ) Plaintiff, ) ) Vo ) ) ROBERT LAZAR, an individual; ) JOY WHITE, an individual; and ) COMPLAINT FOR INJUNCTION UNITED NUCLEAR SCIENTIFIC ) SUPPLIES, LLC, a corporation, ) ) D~d~ts. ) NATURE OF THE CLAIM 1. Plaintiffbrings this action to obtain permanent injunctive relief halting defendants’ violations of the Federal Hazardous Substances Act ("FHSA"), 15 U.S.C. § 1261 et seq. JURISDICTION AND VENUE 2. This Court has jurisdiction over this action pursuant to 15 U.S.C. § 1267(a) and 28 U.S.C. §§ 1331, 1337, and 1345. 3. Venue in this district is proper pursuant to 28 U.S.C. §§ 1391(b) and (c). DEFENDANTS 4. Defendant United Nuclear Scientific Supplies, LLC, (hereinafter referred to as "United Nuclear"), is a limited liability company existing under the laws of New Mexico, with its COMPLAINT FOR INJUNCTION - 1 principal place of business at 45 Eastridge Road, in Edgewood, New Mexico. United Nuclear has been a distributor and retailer of chemicals and other fireworks components. 5. Defendant Robert Lazar is the president and founder of United Nuclear. At all times material to the Complaint, Robert Lazar answered customer questions, took orders fi:om customers, and processed orders. 6. Defendant Joy White is United Nuclear’s accountant. At all times material to the Complaint, Joy White processed customer orders. THE FEDERAL HAZARDOUS SUBSTANCES ACT 7. The U.S. Consumer Product Safety Commission ("CPSC") is an independent federal agency, authorized to administer the FHSA. 15 U.S.C. § 1261 et seq. -

The Following 24 Chemicals Are Explosive Precursors. This Iist Is Not
Name Description Odour Acetic Anhydride Colourless Liquid Vinegar Acetone Colourless Liquid Fruit/Nail Varnish Remover Aluminium Powder Silver/Grey Powder Odourless Ammonium Nitrate Colourless Ammonia Ammonium Perchlorate Grainy White Powder Odourless Barium Nitrate White Crystals (Not Documented) Highly Toxic Calcium Ammonium Nitrate Colourless Liquid Ammonia Guanidine Nitrate Colourless Crystals Odourless Hexamine Waxy Solid Mild Ammonia Hydrogen Peroxide Colourless Liquid Odourless Nitric Acid Colourless, Yellow or Red Crystals Acrid Explosive Precursor Chemicals Nitromethane Colourless Oily Liquid Sweet/Fruit Perchloric Acid Colourless Liquid Odourless EXPLOSIVE Potassium Chlorate White Crystals Odourless Potassium Nitrate White Crystals Odourless Potassium Nitrite Yellow/White Crystals Odourless Potassium Perchlorate White/Colourless Crystals Odourless PRECURSOR Sodium Azide White Crystals Odourless Sodium Chlorate White Crystals Odourless Sodium Nitrate White Crystals Odourless CHEMICALS Sodium Nitrite Yellow/White Crystals Odourless Sodium Perchlorate White Crystals Odourless Sulphuric Acid Colourless Liquid Rotten Eggs The following 24 chemicals are explosive precursors. This Urea White Crystals Ammonia Iist is not extensive, however those that appear below are Acetic Acid White Crystals Vinegar regulated and subject to controls by one or more of the Alcohol (Ethanol, Methanol) Colourless Liquid Odourless national governments and agencies in Canada, USA, EU, UK, Anything Chlorate or Perchlorate Australia and Singapore and raise -

Chemical Incompatibilities
Chemical Incompatibilities Incompatibilities Chromic acid, nitric acid, hydroxyl compounds, ethylene glycol, Acetic acid perchloric acid, peroxides, permanganates Acetylene Chlorine, bromine, copper, fluorine, silver, mercury Acetone Concentrated nitric and sulfuric acid mixtures Alkali and alkaline earth metals (such as Water, carbon tetrachloride or other chlorinated hydrocarbons, powdered aluminum or magnesium, calcium, carbon dioxide, halogens lithium, sodium, potassium) Mercury (in manometers, for example), chlorine, calcium Ammonia (anhydrous) hypochlorite, iodine, bromine, hydrofluoric acid (anhydrous) Acids, powdered metals, flammable liquids, chlorates, nitrites, Ammonium nitrate sulfur, finely divided organic combustible materials Aniline Nitric acid, hydrogen peroxide Arsenical materials Any reducing agent Azides Acids Bromine See chlorine Calcium oxide Water Carbon (activated) Calcium hypochlorite, all oxidizing agents Carbon tetrachloride Sodium Ammonium salts, acids, powdered metals, sulfur, finely divided Chlorates organic or combustible materials Acetic acid, naphthalene, camphor, glycerol, alcohol, flammable Chromic acid and chromium liquids in general Ammonia, acetylene, butadiene, butane, methane, propane (or Chlorine other petroleum gases), hydrogen, sodium carbide, benzene, finely divided metals, turpentine Chlorine dioxide Ammonia, methane, phosphine, hydrogen sulfide Copper Acetylene, hydrogen peroxide Cumene hydroperoxide Acids (organic or inorganic) Cyanides Acids Ammonium nitrate, chromic acid, hydrogen peroxide, -

Reducing the Threat of Improvised Explosive Device Attacks by Restricting Access to Explosive Precursor Chemicals
CISA | CYBERSECURITY AND INFRASTRUCTURE SECURITY AGENCY REDUCING THE THREAT OF IMPROVISED EXPLOSIVE DEVICE ATTACKS BY RESTRICTING ACCESS TO EXPLOSIVE PRECURSOR CHEMICALS Craig Conklin July 17, 2019 Background Mass Event Location Main Charge (lb)* . Terrorists and other malicious actors employ 1970-Sterling Hall Bombing (Madison, WI) # ANFO 2,000 1983-Beirut Barracks Bombing (Beirut, Lebanon) PETN 20,000 1983-US Embassy Bombings (Beirut, Lebanon) % ANFO 2,000 large- and small-scale IEDs 1992-St. Mary Axe Bombing (London, United Kingdom) # CAN/IS 2,000 1993-World Trade Center Bombing (New York, NY) # Urea Nitrate 1,200 1993-Bishopsgate Bombing (London, United Kingdom) # CAN/IS 4,000 1995-Oklahoma City Bombing (Oklahoma City, OK) # AN/NM 5,000 Vehicle-borne IEDs (VBIEDs): ~40-10,000(s) lbs. 1996-Manchester Shopping Mall (Manchester, United Kingdom) # CAN/IS 3,000 1996-South Quay Bombing (London, United Kingdom) # CAN/IS 3,000 1996-Khobar Towers Bombing (Khobar, Saudi Arabia) C4 20,000 Person-borne IEDs (PBIEDs): ~1-40 lbs. 1998-US Embassy Bombings (Tanzania, Kenya) TNT 2,000 1999-Millennial Bomber Interdiction (Port Angeles, WA) # Urea Nitrate 500 2000-USS Cole Bombing (Aden, Yemen) Mil. Exp. 1,000 2001-Shoe Bomber (AA Flight 63) PETN 1 . Materials, devices, and instructions for 2002-Bali Nightclub Bombing (Bali, Indonesia) # KClO3/S/Al 2,000 2003-Marriott Hotel Jakarta Bombing (Jakarta, Indonesia) # KClO3/S/Al 100 2003-Britsh Consulate Bombing (Istanbul, Turkey) # AN/AI 2,000 producing IEDs are highly accessible 2003-Casablanca Bombings (Casablanca, Morocco) # TATP/AN 20 2004-Australian Embassy Attack (Jakarta, Indonesia) # KClO3/S/Al 2,000 2004-US Consulate Failed Attack (Karachi, Pakistan) # CHP/Flour 2,000 2004-Distrupted Jordanian Attack (Amman, Jordan) # CHP/Cumin 10,000 2004-US Embassy Attack (Tashkent, Uzbekistan) # AN/AI 20 . -

The Decomposition of Potassium Chlorate
Experiment 4C FV 1-21-16 THE DECOMPOSITION OF POTASSIUM CHLORATE MATERIALS: test tubes: (25x150 (Instructor Demo, Part A), 18x150 (Part B)); 100 mL beaker, glass wool, potassium chlorate, manganese(IV) oxide. PURPOSE: The purpose of this experiment is to study the decomposition of potassium chlorate and quantitatively determining the correct stoichiometry. LEARNING OBJECTIVES: By the end of this experiment, the student should be able to demonstrate the following proficiencies: 1. Find the MSDS and/or Safety Card for a chemical species, and locate important information related to physical properties, reactivity, and appropriate handling protocols. 2. After carrying out the decomposition reaction for potassium chlorate, quantitatively verify its stoichiometry. DISCUSSION: Stoichiometry. A major emphasis of chemistry is the understanding chemical reactions. This requires knowing the correct formulas for all reactants and products involved in the reaction, as well as the relative molar amounts of each. Such information is provided by the balanced chemical reaction, but where does that come from? The answer is that reactions are determined by experiment. Careful mass measurements and physical and/or chemical tests allow one to deduce the proper reaction. Only when that is understood can one start to consider useful applications of the reaction. Consider the title reaction, the thermal decomposition of potassium chlorate. When KClO3 is heated strongly, it breaks down releasing oxygen gas and leaving behind a thermally stable (i.e., heat-insensitive) solid residue of an ionic potassium compound. solid potassium chlorate oxygen gas + solid residue There are at least three plausible reactions one can write for the process, but only one occurs to any significant extent. -

Chemical Source of Oxygen John F
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Northern Iowa Proceedings of the Iowa Academy of Science Volume 66 | Annual Issue Article 28 1959 Chemical Source of Oxygen John F. O'Brien Saint Ambrose College Copyright © Copyright 1959 by the Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation O'Brien, John F. (1959) "Chemical Source of Oxygen," Proceedings of the Iowa Academy of Science: Vol. 66: No. 1 , Article 28. Available at: https://scholarworks.uni.edu/pias/vol66/iss1/28 This Research is brought to you for free and open access by UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. O'Brien: Chemical Source of Oxygen Chemical Source of Oxygen 1 By JOHN F. O'BRIEN Abstract. An investigation was made on the thermal decom position of the alkali metal chlorates and perchlorates of sodium, potassium and lithium, and mixtures of these. The effects of various catalysts in promoting and controlling the decomposition with the production of minimum reaction temperature and maxi mum oxygen yield were studied. Experimental results are pre sented for potassium chlorate and perchlorate. In recent years the military has advocated the development of a solid chemical oxygen source to the point where it may be used in an aircraft oxygen system. The need exists for a reliable oxygen source that is efficient on a weight and volume basis, and suf ficiently developed for immediate availability for military use.