Equine Pharmacology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Social and Economic Roots of the Scientific Revolution
THE SOCIAL AND ECONOMIC ROOTS OF THE SCIENTIFIC REVOLUTION Texts by Boris Hessen and Henryk Grossmann edited by GIDEON FREUDENTHAL PETER MCLAUGHLIN 13 Editors Preface Gideon Freudenthal Peter McLaughlin Tel Aviv University University of Heidelberg The Cohn Institute for the History Philosophy Department and Philosophy of Science and Ideas Schulgasse 6 Ramat Aviv 69117 Heidelberg 69 978 Tel Aviv Germany Israel The texts of Boris Hessen and Henryk Grossmann assembled in this volume are important contributions to the historiography of the Scientific Revolution and to the methodology of the historiography of science. They are of course also historical documents, not only testifying to Marxist discourse of the time but also illustrating typical European fates in the first half of the twentieth century. Hessen was born a Jewish subject of the Russian Czar in the Ukraine, participated in the October Revolution and was executed in the Soviet Union at the beginning of the purges. Grossmann was born a Jewish subject of the Austro-Hungarian Kaiser in Poland and served as an Austrian officer in the First World War; afterwards he was forced to return to Poland and then because of his revolutionary political activities to emigrate to Germany; with the rise to power of the Nazis he had to flee to France and then America while his family, which remained in Europe, perished in Nazi concentration camps. Our own acquaintance with the work of these two authors is also indebted to historical context (under incomparably more fortunate circumstances): the revival of Marxist scholarship in Europe in the wake of the student movement and the pro- fessionalization of history of science on the Continent. -

Le Costellazioni
ASTRONOMIA VALLI DEL NOCE www.astronomiavallidelnoce.it [email protected] LE COSTELLAZIONI Una costellazione (dal latino constellatio, cum+stellatus) è un gruppo di stelle visibili che assumono una particolare forma sulla volta celeste. Queste forme sono date dalla pura immaginazione, nella realtà le stelle non hanno questa correlazione. Stelle che sulla volta celeste ci appaiono vicine tra loro, nello spazio tridimensionale potrebbero risultare più distanti rispetto a stelle di costellazioni diverse. Ciò che noi vediamo è solo la loro proiezione sulla volta celeste. Il raggruppamento delle costellazioni è essenzialmente arbitrario e può variare da cultura a cultura. Alcune forme più facili da identificare erano comuni a più culture. L'Unione Astronomica Internazionale (UAI) ha diviso il cielo in 88 costellazioni ufficiali con confini precisi, in modo che il cielo sia completamente diviso in settori univoci. Oggi col termine costellazione non si intendono più le figure formate dalle stelle, ma delle aree di cielo ben definite. Storia delle costellazioni Fin dal Paleolitico l'uomo ha guardato il cielo, sia per motivi pratici (orientamento, misurazione del tempo, agricoltura) che religiosi (culti di divinità astrali, interpretazione degli eventi). Nel Paleolitico Superiore (16000 anni fa), l’uomo aveva dato vita ad un sistema di 25 costellazioni, ripartite in tre gruppi, riconducibili metaforicamente alle tre dimensioni con cui tutti i popoli da sempre hanno rappresentato il mondo: il Paradiso, la Terra e gli Inferi: • Primo gruppo: costellazioni riferite al mondo superiore, ovvero dominate da creature aeree (ad es. Cigno, Aquila, Pegaso, ecc.), le quali avevano al culmine la maggior altezza sull’orizzonte; • Secondo gruppo: costellazioni legate alla Terra (ad es. -

Prince Henry the Navigator, Who Brought This Move Ment of European Expansion Within Sight of Its Greatest Successes
This is a reproduction of a library book that was digitized by Google as part of an ongoing effort to preserve the information in books and make it universally accessible. https://books.google.com PrinceHenrytheNavigator CharlesRaymondBeazley 1 - 1 1 J fteroes of tbe TRattong EDITED BY Sveltn Bbbott, flD.B. FELLOW OF BALLIOL COLLEGE, OXFORD PACTA DUOS VIVE NT, OPEROSAQUE OLMIA MHUM.— OVID, IN LI VI AM, f«». THE HERO'S DEEDS AND HARD-WON FAME SHALL LIVE. PRINCE HENRY THE NAVIGATOR GATEWAY AT BELEM. WITH STATUE, BETWEEN THE DOORS, OF PRINCE HENRY IN ARMOUR. Frontispiece. 1 1 l i "5 ' - "Hi:- li: ;, i'O * .1 ' II* FV -- .1/ i-.'..*. »' ... •S-v, r . • . '**wW' PRINCE HENRY THE NAVIGATOR THE HERO OF PORTUGAL AND OF MODERN DISCOVERY I 394-1460 A.D. WITH AN ACCOUNr Of" GEOGRAPHICAL PROGRESS THROUGH OUT THE MIDDLE AGLi> AS THE PREPARATION FOR KIS WORlf' BY C. RAYMOND BEAZLEY, M.A., F.R.G.S. FELLOW OF MERTON 1 fr" ' RifrB | <lvFnwn ; GEOGRAPHICAL STUDEN^rf^fHB-SrraSR^tttpXFORD, 1894 ule. Seneca, Medea P. PUTNAM'S SONS NEW YORK AND LONDON Cbe Knicftetbocftet press 1911 fe'47708A . A' ;D ,'! ~.*"< " AND TILDl.N' POL ' 3 -P. i-X's I_ • •VV: : • • •••••• Copyright, 1894 BY G. P. PUTNAM'S SONS Entered at Stationers' Hall, London Ube ftntcfeerbocfter press, Hew Iffotfc CONTENTS. PACK PREFACE Xvii INTRODUCTION. THE GREEK AND ARABIC IDEAS OF THE WORLD, AS THE CHIEF INHERITANCE OF THE CHRISTIAN MIDDLE AGES IN GEOGRAPHICAL KNOWLEDGE . I CHAPTER I. EARLY CHRISTIAN PILGRIMS (CIRCA 333-867) . 29 CHAPTER II. VIKINGS OR NORTHMEN (CIRCA 787-1066) . -

Namen Van Sterrenbeelden En Sterren
Prof. Dr. Ρ. Η. van Laer VREEMDE WOORDEN IN DE STERRENKUNDE en namen van sterrenbeelden en sterren Tweede herziene druk J. B. Wolters Groningen 1964 Uitgegeven met steun van het Prins Bernhardfonds Inhoud Blz. Voorrede van Prof. Minnaert 5 Verantwoording 7 Lijst van de gebruikte afkortingen en tekens 11 Het Griekse alfabet 12 Transcriptie en uitspraak van Arabische woorden . 13 Uitspraak van de Griekse en Latijnse woorden 14 EERSTE AFDELING Vreemde woorden in de sterrenkunde 19 TWEEDE AFDELING Namen van sterrenbeelden en sterren 71 Historische inleiding 71 Verklaring van de namen van sterrenbeelden en sterren ... 78 Planeten en hun manen 107 Planetoïden 112 De maan 114 Kometen 117 Meteoorzwermen 118 INDICES Nederlandse namen van sterrenbeelden 119 Lijst van de behandelde namen van sterren, planeten, planetoïden en manen 122 3 Voorrede van Prof \ Dr. M. G. /. Minnaert bij de eerste druk Het gebruik van vreemde woorden in de wetenschap heeft zijn kwade, maar ook zijn goede zijde. Het is een euvel, daar het de begrijpelijkheid van de tekst voor den niet-geschoolden lezer bemoeilijkt, de stijl onper- soonlijker en vlakker maakt. Anderzijds is het een voordeel, daar de vast- stelling van internationaal gangbare, nauwkeurig gedefinieerde termen de scherpe vastlegging der begrippen bevordert, en de betrekkingen verge- makkelijkt tussen de wetenschappelijke werkers over de gehele wereld. Het werk van Dr. van Laer nu is bij uitstek geschikt om tegemoet te ko- men aan de bezwaren, en de voordelen tot hun recht te laten komen. Hij brengt ons vooreerst een korte, heldere verklaring van wat elk vreemd woord in onze wetenschap betekent: een belangrijk hulpmiddel dus voor ieder, die zich in de Sterrekunde inwerken wil. -

THE CONSTELLATION LYNX Lynx, Named After the Animal of That Name, Is a Constellation in the Northern Sky That Was Introduced in the 17Th Century by Johannes Hevelius
THE CONSTELLATION LYNX Lynx, named after the animal of that name, is a constellation in the northern sky that was introduced in the 17th century by Johannes Hevelius. This is a faint constellation with its brightest stars forming a zigzag line. The orange giant Alpha Lyncis is the brightest star in the constellation, while the semiregular variable star Y Lyncis is a target for amateur astronomers. Six star systems have been found to contain planets. 6 Lyncis and HD 75898 were discovered to have planets by the Doppler method, while XO-2, XO-4, XO-5 and WASP-13 were found to have planets that were observed as they passed in front of the host star. Within the constellation's borders lie NGC 2419, an unusually remote globular cluster, the galaxy NGC 2770, which has hosted three recent Type Ib supernovae; the distant quasar APM 08279+5255, whose light is magnified and split into multiple images by the gravitational lensing effect of a foreground galaxy; and the Lynx Supercluster, which was the most distant supercluster known at the time of its discovery in 1999. HISTORY Polish astronomer Johannes Hevelius formed the constellation in the 17th century from 19 faint stars that he observed with the unaided eye between the constellations Ursa Major and Auriga. Naming it Lynx because of its faintness, he challenged future stargazers to see it, declaring that only the lynx-eyed (those of good sight) would have been able to recognize it. There is a figure in mythology who might be linked to the constellation’s name. -
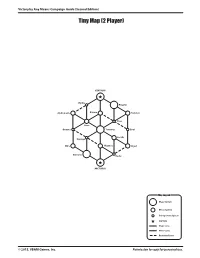
Tiny Map (2 Player)
Victory by Any Means Campaign Guide (Second Edition) Tiny Map (2 Player) CENTAURI Hydra Regulus Andromeda Baham Rotanev Naos Siren Gemma Terminus Errai Lesath Kapteyn Mira Phaeton Algol Canopus Hadar ARCTURUS Map Legend Major System Minor System Unimportant System CAPITAL Major Lane Minor Lane Restricted Lane © 2015, VBAM Games, Inc. Permission to copy for personal use. Victory by Any Means Campaign Guide (Second Edition) Small Map (3 Player) CENTAURI Scorpius Rigel Zaurak Canopus Regulus Sualocin Cassiopeia Perseus Errai Malus Menkar Algol Dorado Chara Bessel Sadatoni Ankaa Pegasus Terminus Cayrel Celaeno Tania Theemin Sabik Aries Vega Sheliak Aquila DRACONIS ORION Phoenix Tigris Herschel Sirius Ksora Aldebaran Map Legend Major System Minor System Unimportant System CAPITAL Major Lane Minor Lane Restricted Lane © 2015, VBAM Games, Inc. Permission to copy for personal use. Victory by Any Means Campaign Guide (Second Edition) Medium Map (4 Player) Canopus Cerberus Betria Vulpecula Pegasus Luyten ORION CENTAURI Sualocin Kapteyn Mintaka Mizar Phaeton Rana Altair Lilium Leo Rangifer Ruchba Thuban Mira Asterion Sabik Ksora Nihal Spica Sulafat Alshat Pavonis Sagittarius Lyra Terminus Capella Sarin Gemma Heka Hercules Hadar Taygeta Bootes Rotanev Geidi Noctua Algol Phoenix Errai Regulus Fomalhaut Tigris Alrischa Aquila Dorado Ankaa ARCTURUS ANTARES Eridanus Lesath Perseus Celaeno Zaurak Map Legend Sirius Major System Minor System Unimportant System CAPITAL Major Lane Minor Lane Restricted Lane © 2015, VBAM Games, Inc. Permission to copy for personal -

52-1 Winter 2015
The Valley Skywatcher Official Publication of the Chagrin Valley Astronomical Society PO Box 11, Chagrin Falls, OH 44022 www.chagrinvalleyastronomy.org Founded 1963 C ONTENTS O F F I C E R S F O R 2 0 1 5 Articles President Marty Mullet Why Are You Here? 2 Vice President Ian Cooper A New Telescope Comes to Indian Hill 2 Treasurer Steve Fishman Regular Features Secretary Christina Gibbons Astrophotography 4 Observer’s Log 8 Directors of Observations Bob Modic Mike Hambrecht President’s Corner 11 Constellation Quiz 11 Observatory Director Ray Kriedman Reflections 12 Historian Dan Rothstein Notes & News 13 Editor Ron Baker Lunar Eclipse on October 8, 2014 at 6:54 am EDT. Photo by CVAS member Steve Fishman The Valley Skywatcher • Winter 2015 • Volume 52-1 • Page 1 Why are you here? By Marty Mullet One evening last year, I was sitting on my deck absent- and introduce them to astronomy. Over the years, my mindedly scanning the stars when my eye caught the telescopes got bigger (as did my waistline), and my Great Square of Pegasus rising in the east. I’d seen the observing skills improved. I discovered CVAS and my Great Square countless times before, but for some spark became a blaze. I found myself enjoying reason on this occasion my mind went back to 1980 and outreach and public events, and I began sharing the the first time I’d noticed it. That’s when it hit me: These eyepiece with others, explaining what little I know and four stars that looked like a baseball diamond first lit the learning from those who know more. -

Latin and the Vernaculars in Early Modern Europe Ed
Tidsskrift for renæssanceforskning 6 2010 Latin and the Vernaculars in Early Modern Europe ed. Trine Arlund Hass & Johann Ramminger LATIN AND THE VERNACULARS IN EARLY MODERN EUROPE Renæssanceforum 6 • 2010 • www.renaessanceforum.dk Preface The Latin/vernacular bilingualism of early modern culture is a phenomenon which only in recent years has begun to attract serious scholarly attention. The dynamics of the multilingual culture of early modern Europe go from rivalry to cross-fertilisation, from an agenda of defence of Latin – or matter- of-fact statements of the superiority of the Latin language – and newly found assertiveness of the vernaculars to concerted bilingual strategies of propaganda and outreach. The studies assembled in this volume throw spot- lights on a diverse array of factors in play in the multilingual culture of early modern Europe. The Italian humanists of the Quattrocento trying to come to grips with the bilingualism of their culture had to develop a theoretical framework and a Latin terminology for the relationship between Latin and the volgare, since – even though some humanists believed that ancient Rome had already been bilingual – such phenomena were discussed, if at all, only indirectly in classical literature. Ramminger’s contribution examines the spectacular rise in the fifteenth century of the central terminus technicus, the word ver- naculus, also used in the title of this collection. A century later, the situation had changed dramatically. In the literary landscape of sixteenth-century Italy Latin was inexorably receding against Tuscan in the hierarchy of languages. Laureys brings forward the little known Pro lingua Latina of Gabriel Barrius, which tries to shore up support for Latin by emphasizing both its international importance, its preeminence over all other languages as the language of the Christian faith, and (by arguing for a muted form of Ciceronianism) its versatility. -

Constellations Concept Booklet Dan Lovallo 1 Constellations Algorithm A1) Selection of Constellation in Relation to Location: E.G
constellations concept booklet dan lovallo 1 constellations algorithm a1) Selection of constellation in relation to location: e.g. - Select constellations between Ecliptic and Celestial Equator - Number of constellation selection: 4 a2) Extraction of constellation in accordance to original default orientations CANCER a3) Extraction of constellation into vectorial points a1 a2 a3 b1) Overlapping of 4 sets of constellation points above one another. Sequence dependent on Constellation chart. b2) Vertical distances between each layer of constellation points relates to the relative real scale positions of those constellations. b3) Connection of points through single polyline to define total sequencing of constellation points. b1 b2 b3 c1) Panelling in accordance to the connected lines. Each panel is triangulated, governed by the closest two lines which form the edges of the panel. c2) Removal of panel based on random algorithm, which simulates the random connectivity of the constellations’ relative geographical locations. c3) Orientation of final output - select horizontal or vertical. c1 c2 c3 2 night sky photograph 3 visible stars chart 4 official constellations chart 5 chinese constellations chart Dunhuang Star map is one of the first known graphical representation of stars from ancient Chinese astronomy, dated to the Tang Dynasty (618–907) 6 chinese constellations chart 28 Chinese Constellations (asterisms) 7 chinese constellations chart 28 Chinese Constellations (asterisms) 8 egyptian constellations chart the zodiacal and para-zodiacal -

The Art of Memory
FRANCES YATES Selected Works Volume III The Art of Memory London and New York FRANCES YATES Selected Works VOLUME I The Valois Tapestries VOLUME II Giordano Bruno and the Hermetic Tradition VOLUME III The Art of Memory VOLUME IV The Rosicrucian Enlightenment VOLUME V Astraea VOLUME VI Shakespeare's Last Plays VOLUME VII The Occult Philosophy in the Elizabethan Age VOLUME VIII Lull and Bruno VOLUME IX Renaissance and Reform: The Italian Contribution VOLUME X Ideas and Ideals in the North European Renaissance First published 1966 by Routledge & Kcgan Paul Reprinted by Routledge 1999 11 New Fetter Lane London EC4I' 4EE Simultaneously published in the USA and Canada by Routledge 29 West 35th Street, New York, NY 10001 Routledge is an imprint of the Taylor & Francis Croup © 1966 Frances A. Yates Printed and bound in Great Britain by Antony Rowe Ltd, Chippenham, Wiltshire Publisher's note The publisher has gone to great lengths to ensure the quality of this reprint but points out that some imperfections in the original book may be apparent. British Library Cataloguing in Publication Data A CIP record of this set is available from the British Library Library of Congress Cataloging in Publication Data A catalogue record for this book has been requested ISBN 0-415-22046-7 (Volume 3) 10 Volumes: ISBN 0-415-22043-2 (Set) Hermetic Silence. From Achilles Bocchius, Symbolicarum quaestionum . libri quinque, Bologna, 1555. Engraved by G. Bonasone (p. 170) FRANCES A.YATES THE ART OF MEMORY ARK PAPERBACKS London, Melbourne and Henley First published in 1966 ARK Edition 1984 ARK PAPERBACKS is an imprint of Routledgc & Kcgan Paul plc 14 Leicester Square, London WC2II 7PH, Kngland. -

Pseudo-Mâshâ'allâh on the Astrolabe
Pseudo-Mâshâ’allâh On the Astrolabe Part VI: English Translation by Ron B. Thomson Version 1.6 Toronto, 2020 © copyright 2012, 2014, 2015, 2016, 2018, 2019, 2020 by Ron B. Thomson COPYRIGHT CONDITIONS Any individual may (A) print out any or all of the pages of this work for personal study, (B) bind this print-out in book form, and/or (C) quote from or cite from it following the normal rules of fair dealing. No one may (A) produce a copy (of all or part of the work) for a fee or for sale, (B) produce multiple copies of all or parts of this work, and/or (C) sell copies of this work without the permission of the copyright owner. Copyright extends for the life of the author/editor, and for another 75 years after that (during which time copyright is administered by the author’s estate/heirs/executor). Proper citation of this work: Pseudo-Mâshâ’allâh, On the Astrolabe, ed. Ron B. Thomson, version 1.6 (Toronto, 2020) [ ON THE ASTROLABE: CONSTRUCTION ] [Prologue] Here begins the astrolabe [text] of Messehalle / Prologue to the astrolabe [text] of Messehalle 2 [Construction, Section I] First chapter On the construction of an astrolabe: On the preparation of the mother 3 [Chapter 2.] On the back side of the astrolabe; and first the circle of altitude 5 [Chapter 3.] The method of the engraving of the shadow square 10 [Chapter 4.] On the fabrication of the common alidade, which is also called the rule 11 [Chapter 5.] On the arrangement of the hours on the rule, which is also called the “time-telling” alidade 14 [Chapter 5 BIS.] On the fabrication -

Giordano Bruno : His Life, Thought, and Martyrdom
GIORDANO BRUNO BY THE SAME AUTHOR A HISTORY OF THE ITALIAN REPUBLICS IN THE MIDDLE AGES. By J. C. L. SISMONDI. Translated, supple mented and edited, with a Memoir of the Author, by Dr. WILLIAM BOULTING. Demy 8vo, buckram, 5J-. net. GIORDANO BRUN HIS LIFE, THOUGHT, AND MARTYRDOM BY WILLIAM BOULTING " AUTHOR OF TASSO AND HIS TIMES" SYLVIUS," ETC. " To love Truth for Truth s sake is the principal part of human perfection in this world, and the seed-plot of all other virtues." LOCKE. LONDON KEGAN PAUL, TRENCH, TRtTBNER & CO. L BROADWAY HOUSE, 68-74 CARTER LANE, E.G. 1914 The rights of translation and of reproduction are reserved Printed by BALLANTYNK, HANSON &* Co. at the Ballantyne Press, Edinburgh PREFACE THE life of Bruno has been written in English more than once, and he has had many excellent commentators. No one can now write about him without availing himself very largely of the solid and scholarly work done by Tocco, Fio- rentino, Berti, Brunnhofer, Mclntyre and others. The apology for the appearance of the present work must be that some facts, unrecorded in England, have come to light of late years, and also that a few usual, almost unavoidable in accuracies require correction. Moreover, I have tried to follow the development of Bruno s thoughts in the order in or I find which he declared them ; and, on one two points, myself compelled to differ from the conclusions of this or the other commentator. Most of Bruno s works have been published in the editions of Wagner, Lagarde, and Gfrorer, well edited for their day.