Torus-Bearing Pit Membranes in Species of Osmanthus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytophthora Ramorum Sudden Oak Death Pathogen
NAME OF SPECIES: Phytophthora ramorum Sudden Oak Death pathogen Synonyms: Common Name: Sudden Oak Death pathogen A. CURRENT STATUS AND DISTRIBUTION I. In Wisconsin? 1. YES NO X 2. Abundance: 3. Geographic Range: 4. Habitat Invaded: 5. Historical Status and Rate of Spread in Wisconsin: 6. Proportion of potential range occupied: II. Invasive in Similar Climate YES NO X Zones United States: In 14 coastal California Counties and in Curry County, Oregon. In nursery in Washington. Canada: Nursery in British Columbia. Europe: Germany, the Netherlands, the United Kingdom, Poland, Spain, France, Belgium, and Sweden. III. Invasive in Similar Habitat YES X NO Types IV. Habitat Affected 1. Habitat affected: this disease thrives in cool, wet climates including areas in coastal California within the fog belt or in low- lying forested areas along stream beds and other bodies of water. Oaks associated with understory species that are susceptible to foliar infections are at higher risk of becoming infected. 2. Host plants: Forty-five hosts are regulated for this disease. These hosts have been found naturally infected by P. ramorum and have had Koch’s postulates completed, reviewed and accepted. Approximately fifty-nine species are associated with Phytophthora ramorum. These species are found naturally infected; P. ramorum has been cultured or detected with PCR but Koch’s postulates have not been completed or documented and reviewed. Northern red oak (Quercus rubra) is considered an associated host. See end of document for complete list of plant hosts. National Risk Model and Map shows susceptible forest types in the mid-Atlantic region of the United States. -

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

GREENING ROME the Urban Green of the Metropolitan Area of Rome in the Context of the Italian MAES Process
GREENING ROME The Urban Green of the Metropolitan Area of Rome in the Context of the Italian MAES Process Italian Ministry for the Environment and the Protection of Land and Sea - Maria Carmela Giarratano - Eleonora Bianchi - Eugenio Dupré Italian Botanical Society - Scientific Coordination: Carlo Blasi - Working Group: Marta Alós, Fabio Attorre, Mattia Martin Azzella, Giulia Capotorti, Riccardo Copiz, Lina Fusaro, Fausto Manes, Federica Marando, Marco Marchetti, Barbara Mollo, Elisabetta Salvatori, Laura Zavattero, Pier Carlo Zingari 1. Introduction The Italian Ministry for the Environment provides financial support to academia (Italian Botanical Society – SBI and Italian Zoological Union – UZI) for the implementation of the MAES process in Italy. A preliminary collection of updated and detailed basic data at the national level was carried out, including ecoregions, land units, bioclimate, biogeography, potential natural vegetation and CORINE land cover at the fourth level. Starting from these data, the Italian MAES process has been organised into six steps. The outcomes of this process have provided the Ministry for the Environment with a reliable body of information targeted to: - an effective implementation of the National Biodiversity Strategy (MATTM 2010); - the improvement in biodiversity data within the National Biodiversity Network (Martellos et al. 2011); - the further development of the environmental accounting system (Capotorti et al. 2012b); - the implementation of a road-map to be delivered to the regions (sub-national level); - the effective use of the products of all MAES process to concretely support European structural and investment funds 2014-2020 through the Joint Committee on Biodiversity, the governing body of the Italian National Strategy (Capotorti et al., 2015). -

Osmanthus Fragrans
Osmanthus fragrans (Sweet Osmanthus, fragrant Tea olive) Sweet Tea Olive is large evergreen shrub or small tree is capable of reaching 6-8 m in height and width but is most often seen at 3-4 m high with a 2 m spread. The shiny, medium-green leaves have paler undersides and are joined from October through March by a multitude of small, but extremely fragrant, white blossoms. They perfume a large area of the landscape and can be showy in some years. The plant has a upright oval to columnar growth habit in youth. Sweet Osmanthus is ideal for use as an unclipped hedge or trained as a small tree, and should be placed where its fragrance can be enjoyed. Since the flowers are not particularly showy, people will wonder where the delightful fragrance is coming from. Sweet Osmanthus should be grown in full sun or partial shade in well-drained soil. Plants are fairly drought-tolerant once established but will perform their best with ample moisture. Needs acidic soil to grow in. Landscape Information French Name: Osmanthe fragrante, Osmanthe parfumée Pronounciation: oz-MANTH-us FRAY-granz Plant Type: Shrub Origin: Japan, China Heat Zones: 8, 9, 10, 11, 12 Hardiness Zones: 7, 8, 9, 10, 11 Uses: Screen, Hedge, Container Size/Shape Growth Rate: Slow Tree Shape: Round Canopy Symmetry: Symmetrical Canopy Density: Medium Canopy Texture: Medium Height at Maturity: 3 to 5 m Spread at Maturity: 3 to 5 meters Plant Image Osmanthus fragrans (Sweet Osmanthus, fragrant Tea olive) Botanical Description Foliage Leaf Arrangement: Opposite Leaf Venation: Pinnate Leaf -

Upland & Pollinator Plants Flip Book
Pictures Ilex opaca Callicarpa americana American Holly Beautyberry Prunus serotina Pteridium aquilinum Black Cherry Bracken Fern Descriptions Callicarpa americana Ilex opaca The American beautyberry is a shrub, 6-9 ft in height. Shoot This tree grows to be 35 to 50 ft tall and 15 to 25 ft wide. growth occurs throughout much of the season. It is It has a dense, pyramid-shaped crown. characterized by its attractive foliage and clusters of flowers or fruit around the leaf nodes. Leaves Leaves Alternate, simple and laceolate or elliptic in shape, 2-4 inches long and 1-1.5 inches wide. Medium to dark, Simple, opposite or subopposite, and deciduous. 3-5” long green in color, and shiny or flat. Leaf margins are usually and 1-3” wide, oval shaped, with an acute apex. Leaf bases spiny and will be flat or wavy. are tapered, margins are serrate. Surfaces are green and usually glabrous above, paler and pubescent below. Flowers Petioles are short and slender. Small, green or white in color, fragrant and found in Flowers clusters in the leaf axils. Perfect, in sessile clusters around the leaf nodes Bark Fruit The bark is light gray and may be covered by wart-like growths. Because the bark is very thin, it is easily A 4-pitted lavender-pink, magenta, or violet drupe, about 1/4 damaged. inch in diameter. Lasts long into winter and is eaten by a variety of birds. Interesting Facts Trunk The preserved berries of this tree were used by American Indians as decorative buttons. Historically, the Older stems have a thin protective, light-brown bark. -

Variety Identification and Landscape Application of Osmanthus Fragrans
l of Hortic na ul r tu u r Mu et al., J Hortic 2019, 6:1 o e J Journal of Horticulture DOI: 10.4172/2376-0354.1000251 ISSN: 2376-0354 ResearchRapid Communication Article OpenOpen Access Access Variety Identification and Landscape Application of Osmanthus fragrans in Jingzhou City Hongna Mu, Fei Zhao, Dongling Li and Taoze Sun* College of Horticulture and Gardening, Yangtze University, No. 88 Jingmi Road, Jingzhou, Hubei 434025, China Abstract Osmanthus fragrans had been considered one of the most popular trees in China for its economic value and cultural significance. O. fragrans, the most ubiquitous garden plants, which varieties had been unclear in Jingzhou city. It is an urgent task to figure out the precise varieties. Therefore, the investigation was carried out. Finally, 24 varieties were identifiedand landscape application had been illustrated in this paper. Keywords: Osmanthus fragrans; Variety identification; Landscape Material and Method application Materials Introduction This study was carried out in Three Kingdoms Park, Mingyue Park, Osmanthus fragrans, commonly known as sweet osmanthus, is Binjiang Park, Zhongshan Park, four typical public parks considering a woody, evergreen species of shrubs and small trees in the Oleaceae the park size, popularity, Vegetation coverage and the numbers of family. Sweet osmanthus has considerable economic value and cultural sweet osmanthus tree. Its distribution was illustrated in Figure 1a-d. significance and is one of the top ten traditional flowers in China. Method of variety survey Twenty-four of the thirty-five species in the Osmanthus genus are distributed in China, with the mostly highly representative species being Prepare variety investigation record card according to the O. -
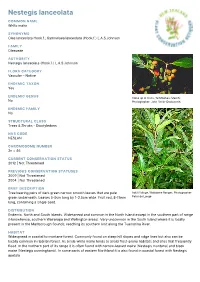
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

Report of Two Spontaneous, Rare Phenotypic Traits in the Genus Phillyrea L
Flora Montiberica 70: 80-86 (III-2018). ISSN: 1138-5952, edic. digital: 1988-799X REPORT OF TWO SPONTANEOUS, RARE PHENOTYPIC TRAITS IN THE GENUS PHILLYREA L. José Luis MEDINA-GAVILÁN SOCEAMB, Sociedad de Estudios Ambientales. C/Perú, 4, 1ª planta. 41100-Coria del Río (Sevilla). [email protected] ABSTRACT: In this note, I document two previously unreported, spontane- ous and exceptionally rare phenotypic expressions affecting reproductive traits in adult plants of the Mediterranean genus Phillyrea (Oleaceae): (i) a morph with an abnormally elongated stigma and lobes transformed in two long branches (i.e. deeper-stigma phenotype), detected in a population of Phillyrea latifolia L. from NE Spain, and (ii) a morph with fruits lacking anthocyanins (i.e. colourless-fruit phenotype), in a population of Phillyrea angustifolia L. from SW Spain. Both phenotypes occurred at a very low frequency within their respective populations. Despite this, the novel traits acquired are discussed in an eco-evolutionary con- text, revealing their potential use as a study model, limited but suggestive, to test adaptive hypothesis in natural conditions. Keywords: phenotypic expression; adaptive value; anemophilous syndrome; selection mediated by frugivorous birds. RESUMEN: Descripción de dos caracteres fenotípicos, raros y espontáneos en el género mediterráneo Phillyrea L. En esta nota documento la presencia de dos expresiones fenotípicas desconocidas, espontáneas y excepcionalmente raras, que afectan a caracteres reproductivos en plantas adultas del género mediterráneo Phillyrea (Oleaceae): (i) un morfo con un estigma anormalmente largo y lóbulos transformados en dos ramas (i.e. fenotipo de estigma profundo), detectado en una población de Phillyrea latifolia L. en el noreste de España, y (ii) un morfo con frutos carentes de antocianinas (i.e. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Structural Diversity and Contrasted Evolution of Cytoplasmic Genomes in Flowering Plants :A Phylogenomic Approach in Oleaceae Celine Van De Paer
Structural diversity and contrasted evolution of cytoplasmic genomes in flowering plants :a phylogenomic approach in Oleaceae Celine van de Paer To cite this version: Celine van de Paer. Structural diversity and contrasted evolution of cytoplasmic genomes in flowering plants : a phylogenomic approach in Oleaceae. Vegetal Biology. Université Paul Sabatier - Toulouse III, 2017. English. NNT : 2017TOU30228. tel-02325872 HAL Id: tel-02325872 https://tel.archives-ouvertes.fr/tel-02325872 Submitted on 22 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REMERCIEMENTS Remerciements Mes premiers remerciements s'adressent à mon directeur de thèse GUILLAUME BESNARD. Tout d'abord, merci Guillaume de m'avoir proposé ce sujet de thèse sur la famille des Oleaceae. Merci pour ton enthousiasme et ta passion pour la recherche qui m'ont véritablement portée pendant ces trois années. C'était un vrai plaisir de travailler à tes côtés. Moi qui étais focalisée sur les systèmes de reproduction chez les plantes, tu m'as ouvert à un nouveau domaine de la recherche tout aussi intéressant qui est l'évolution moléculaire (même si je suis loin de maîtriser tous les concepts...). Tu as toujours été bienveillant et à l'écoute, je t'en remercie. -

Breeding Systems and Reproduction of Indigenous Shrubs in Fragmented
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Breeding systems and reproduction of indigenous shrubs in fragmented ecosystems A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy III Plant Ecology at Massey University by Merilyn F Merrett .. � ... : -- �. � Massey University Palrnerston North, New Zealand 2006 Abstract Sixteen native shrub species with various breeding systems and pollination syndromes were investigated in geographically separated populations to determine breeding systems, reproductive success, population structure, and habitat characteristics. Of the sixteen species, seven are hermaphroditic, seven dioecious, and two gynodioecious. Two of the dioecious species are cryptically dioecious, producing what appear to be perfect, hermaphroditic flowers,but that functionas either male or female. One of the study species, Raukauaanomalus, was thought to be dioecious, but proved to be hermaphroditic. Teucridium parvifolium, was thought to be hermaphroditic, but some populations are gynodioecious. There was variation in self-compatibility among the fo ur AIseuosmia species; two are self-compatible and two are self-incompatible. Self incompatibility was consistent amongst individuals only in A. quercifolia at both study sites, whereas individuals in A. macrophylia ranged from highly self-incompatible to self-compatible amongst fo ur study sites. The remainder of the hermaphroditic study species are self-compatible. Five of the species appear to have dual pollination syndromes, e.g., bird-moth, wind-insect, wind-animal. High levels of pollen limitation were identified in three species at fo ur of the 34 study sites. -

Biological Fire Prevention Method: Evaluating the Effects of Goat Grazing on the Fire-Prone Mediterranean Scrub J
Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA) Forest Systems 2012 21(2), 199-204 Available online at www.inia.es/forestsystems ISSN: 2171-5068 http://dx.doi.org/10.5424/fs/2012212-02289 eISSN: 2171-9845 Biological fire prevention method: Evaluating the effects of goat grazing on the fire-prone mediterranean scrub J. M. Mancilla-Leytón* and A. Martín Vicente Dep. Biología Vegetal y Ecología. Universidad de Sevilla. Apdo.1095 Sevilla 41080 Abstract The effect of goat grazing on the shrubby understory of a pine forest in Doñana Natural Park was evaluated using non-destructive measures of vegetation volume over a period of twenty-four months. After establishing grazing exclu- sion fenced plots 350 adult Payoyas goats were introduced. Vegetation was sampled before the introduction of goats and afterwards twice a year, using the point intercept method and thereby obtaining data of height, frequency, cover and biovolume of species. After two years the total biovolume of the vegetation of the ungrazed area had increased signifi- cantly by 32.9%, while at grazed area, vegetation biovolume decreased significantly by 23.1%, leading to a significant decrease in mean height of the species. Although the number of species remained unchanged throughout the study, significant changes in their relative abundance were found in grazed area. The different responses of scrub species to grazing can be used as a tool to control species sensitive to grazing in shrubby forested areas. Significant reduction of total biovolume due to a reduction in vegetation height will help to reduce fire risk, thus contributing to the conserva- tion of Mediterranean woodlands and forests while also fulfilling an important role in the economic and social lives of the rural population of Mediterranean countries.