To Obtain Approval to Release New Organisms (Through Importing for Release Or Releasing from Containment)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
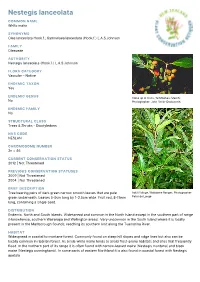
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

Torus-Bearing Pit Membranes in Species of Osmanthus
IAWA Journal, Vol. 31 (2), 2010: 217–226 TORUS-BEARING PIT MEMBRANES IN SPECIES OF OSMANTHUS Roland Dute1, 5, David Rabaey2, John Allison1 and Steven Jansen3, 4 SUMMARY Torus thickenings of pit membranes are found not only in gymnosperms, but also in certain genera of dicotyledons. One such genus is Osmanthus. Wood from 17 species of Osmanthus was searched for tori. Fourteen spe- cies from three of the four sections investigated possessed these thick- enings. Ten of the species represent new records. Only the three New Caledonian species of Section Notosmanthus lacked tori. This observation in combination with other factors serves to isolate this section from the remainder of the genus. Key words: Notelaea, Osmanthus, pit membrane, torus. INTRODUCTION Osmanthus is a member of the Oleaceae (Olive Family), a moderate-sized family with circa 24 genera and 615 spp. (Stevens 2001). There are 33 natural species within the genus (Table 1; Xiang et al. 2008), and they show a tropical to temperate distribution (Bailey & Bailey 1976; Denk et al. 2001; Xiang et al. 2008). Generally, individual shrubs are found in moist forests (Hsieh et al. 1998; Chou et al. 2000; Denk et al. 2001), frequently on steep slopes (Green 1958). In some instances populations are sub- ject to monsoon conditions and hence to periods of relatively low rainfall (Yang et al. 2008; Hua 2008). Tori are thickenings of intervascular pit membranes found not only in gymnosperm, but also in angiosperm wood, including that of Osmanthus (Dute et al. 2008; Dute et al. 2010). Tori were first observed in O. fragrans, O. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Structural Diversity and Contrasted Evolution of Cytoplasmic Genomes in Flowering Plants :A Phylogenomic Approach in Oleaceae Celine Van De Paer
Structural diversity and contrasted evolution of cytoplasmic genomes in flowering plants :a phylogenomic approach in Oleaceae Celine van de Paer To cite this version: Celine van de Paer. Structural diversity and contrasted evolution of cytoplasmic genomes in flowering plants : a phylogenomic approach in Oleaceae. Vegetal Biology. Université Paul Sabatier - Toulouse III, 2017. English. NNT : 2017TOU30228. tel-02325872 HAL Id: tel-02325872 https://tel.archives-ouvertes.fr/tel-02325872 Submitted on 22 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REMERCIEMENTS Remerciements Mes premiers remerciements s'adressent à mon directeur de thèse GUILLAUME BESNARD. Tout d'abord, merci Guillaume de m'avoir proposé ce sujet de thèse sur la famille des Oleaceae. Merci pour ton enthousiasme et ta passion pour la recherche qui m'ont véritablement portée pendant ces trois années. C'était un vrai plaisir de travailler à tes côtés. Moi qui étais focalisée sur les systèmes de reproduction chez les plantes, tu m'as ouvert à un nouveau domaine de la recherche tout aussi intéressant qui est l'évolution moléculaire (même si je suis loin de maîtriser tous les concepts...). Tu as toujours été bienveillant et à l'écoute, je t'en remercie. -

Breeding Systems and Reproduction of Indigenous Shrubs in Fragmented
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Breeding systems and reproduction of indigenous shrubs in fragmented ecosystems A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy III Plant Ecology at Massey University by Merilyn F Merrett .. � ... : -- �. � Massey University Palrnerston North, New Zealand 2006 Abstract Sixteen native shrub species with various breeding systems and pollination syndromes were investigated in geographically separated populations to determine breeding systems, reproductive success, population structure, and habitat characteristics. Of the sixteen species, seven are hermaphroditic, seven dioecious, and two gynodioecious. Two of the dioecious species are cryptically dioecious, producing what appear to be perfect, hermaphroditic flowers,but that functionas either male or female. One of the study species, Raukauaanomalus, was thought to be dioecious, but proved to be hermaphroditic. Teucridium parvifolium, was thought to be hermaphroditic, but some populations are gynodioecious. There was variation in self-compatibility among the fo ur AIseuosmia species; two are self-compatible and two are self-incompatible. Self incompatibility was consistent amongst individuals only in A. quercifolia at both study sites, whereas individuals in A. macrophylia ranged from highly self-incompatible to self-compatible amongst fo ur study sites. The remainder of the hermaphroditic study species are self-compatible. Five of the species appear to have dual pollination syndromes, e.g., bird-moth, wind-insect, wind-animal. High levels of pollen limitation were identified in three species at fo ur of the 34 study sites. -

Vascular Plants of an Unclassified Islet, Cape Brett Peninsula, Northern New Zealand, by E.K. Cameron, P
TANE 28,1982 VASCULAR PLANTS OF AN UNCLASSIFIED ISLET, CAPE BRETT PENINSULA, NORTHERN NEW ZEALAND by E.K. Cameron Department of Botany, University of Auckland, Private Bag, Auckland SUMMARY Seventy indigenous and 2 adventive vascular plants taxa are recorded for the "unmodified" islet. Its botanical value exceeds its small size because of the modification of the adjacent Cape Brett Peninsula and nearby islands. INTRODUCTION The islet is situated only a few metres off the northern coastline of Cape Brett Peninsula (Fig. 1). This steep beehive-shaped greywacke islet, less than two hectares in area, supports an excellent cover of indigenous vegetation compared with the adjacent goat (Copra hircus) browsed mainland. Approximately thirty minutes was spent on the islet during a four day botanical survey of Cape Brett Peninsula carried out for the Department of Lands and Survey, Auckland, in June 1980 (Cameron 1980). Time permitted only a single south-west to north-east traverse, returning to the starting point via the north-west littoral. PLANT COMMUNITIES For ease of description four plant associations (Fig. 2) are recognised although it must be remembered that these are by no means distinct as they grade into one another. Area 1: Coastal Rock. The amount of coastal rock on the islet is proportional to the degree of wave exposure and thus the north-eastern side of the islet has the greatest amount of exposed rock. Plants such as Asplenium flaccidum ssp. haurakiense, Samolus repens and the shore lobelia (Lobelia anceps) are frequently found growing in cracks and crevices. Others found here include the N.Z. -

9:00 Am PLACE
CARTY S. CHANG INTERIM CHAIRPERSON DAVID Y. IGE BOARD OF LAND AND NATURAL RESOURCES GOVERNOR OF HAWAII COMMISSION ON WATER RESOURCE MANAGEMENT KEKOA KALUHIWA FIRST DEPUTY W. ROY HARDY ACTING DEPUTY DIRECTOR – WATER AQUATIC RESOURCES BOATING AND OCEAN RECREATION BUREAU OF CONVEYANCES COMMISSION ON WATER RESOURCE MANAGEMENT STATE OF HAWAII CONSERVATION AND COASTAL LANDS CONSERVATION AND RESOURCES ENFORCEMENT DEPARTMENT OF LAND AND NATURAL RESOURCES ENGINEERING FORESTRY AND WILDLIFE HISTORIC PRESERVATION POST OFFICE BOX 621 KAHOOLAWE ISLAND RESERVE COMMISSION LAND HONOLULU, HAWAII 96809 STATE PARKS NATURAL AREA RESERVES SYSTEM COMMISSION MEETING DATE: April 27, 2015 TIME: 9:00 a.m. PLACE: Department of Land and Natural Resources Boardroom, Kalanimoku Building, 1151 Punchbowl Street, Room 132, Honolulu. AGENDA ITEM 1. Call to order, introductions, move-ups. ITEM 2. Approval of the Minutes of the June 9, 2014 N atural Area Reserves System Commission Meeting. ITEM 3. Natural Area Partnership Program (NAPP). ITEM 3.a. Recommendation to the Board of Land and Natural Resources approval for authorization of funding for The Nature Conservancy of Hawaii for $663,600 during FY 16-21 for continued enrollment in the natural area partnership program and acceptance and approval of the Kapunakea Preserve Long Range Management Plan, TMK 4-4-7:01, 4-4-7:03, Lahaina, Maui. ITEM 3.b. Recommendation to the Board of Land and Natural Resources approval for authorization of funding for The Nature Conservancy of Hawaii for $470,802 during FY 16-21 for continued enrollment in the natural area partnership program and acceptance and approval of the Pelekunu Long Range Management Plan, TMK 5-4- 3:32, 5-9-6:11, Molokai. -

Rats – Another Mammalian Browser of Tupeia Antarctica? Bec Stanley
Acknowledgements Thanks to Ross Beever, Ewen Cameron, Alan Esler, Peter de Lange and Dick Veitch information and recollections. References Bartlett, J. K.; Gardner, R. O. 1983. Flora of Great Barrier Island. Auckland Botanical Society. McGlone, M. S. 1985. Plant biogeography and the late Cenozoic history of New Zealand. N. Z. Journal of Botany 23: 723-749. The Tree Council 2002. Notable trees of Auckland. Wardle, J. A. 1970. The ecology of Nothofagus solandri. 1. The distribution and relationship with other major forest and scrub species. New Zealand Journal of Botany 8: 494-531. Wardle, J. A. 1984. The New Zealand beeches. N. Z. Forest Service. Wilcox, M. D.; Ledgard, N. J. 1983. Provenance variation in the New Zealand species of Nothofagus. N.Z. Journal of Ecology 6: 19-31. Rats – another mammalian browser of Tupeia antarctica? Bec Stanley Leafy Mistletoes in Auckland coastal maire with Tupeia attached. Since then Jonathan Boow, Cameron Kilgour, George Wilson and Auckland has three extant native leafy mistletoes (of myself have found more each year with the total now five extant leafy species in total native to NZ): Peraxilla standing at 11 coastal maire trees with Tupeia tetrapetala, Ileostylus micranthus and Tupeia parasitic on them. antarctica (de Lange, 1997). Peraxilla tetrapetala is now restricted to Hauturu (Little Barrier Island). The Tupeia are restricted to one valley on the island Ileostylus is the only mistletoe we still have on the which is primarily filled with mixed coastal broadleaf mainland of Auckland (see Cameron 2000). Tupeia forest including large puriri (Vitex lucens), houpara was last reported in this Journal as extinct in Auckland (Pseudopanax lessonii), tawapou (Pouteria costata), (Stanley 1998), but has now been re-discovered on karaka (Corynocarpus laevigatus), kohekohe Motukino (Fanal Island) in the Mokohinau Island Group (Dysoxylum spectabile), mahoe (Melicytus ramiflorus), in the outer Hauraki Gulf. -

Summary of Responses
RESPONSE TO REQUEST FOR GENERAL CONSULTATION ON APP202262 (2014) A range of organisations were contacted and asked if they wished to comment on the proposal to introduce the privet lace bug, Leptophya hospita, as a biological control agent for privets. Responses were received from a range of sources: • Department of Conservation • Environmental organisations and the general public • Non-governmental organisations • Primary production organisations • Regional and Unitary Councils and Territorial Authorities DEPARTMENT OF CONSERVATION David Havell, Technical Advisor (Threats), Department of Conservation There are at least 9 species of privet in New Zealand, (http://www.virtualherbarium.org.nz). DOC records indicate that at least 5 species of privet occur on public conservation land: Chinese privet, Ligustrum sinense; Tree privet, Ligustrum lucidum; Common privet, Ligustrum vulgare; Japanese privet Ligustrum japonicum; and privet, Ligustrum ovalifolium (DOC national plant database 2014). Tree privet has the highest DOC weediness score of the privets, (32) which is higher than either pampas (28), pines (26 to 30), and moth plant(31). Chinese privet has a weediness score of 25, which is similar to black wattle. The other privets have relative low weediness scores, ( 23) or have not been assessed. Privets occur extensively over the landscape of lowland Waikato, bay of Plenty, Auckland and Northland, and are common along transport corridors, disturbed sites, along bush edges and in disturbed lowland forest. Privets can occur either as thickets, hedges, tall trees or as under-storey trees, (personal observation). The National Biocontrol Collective and Landcare Research – Manaaki Whenua propose to introduce a privet biocontrol agent, Privet lace bug, Leptophya hospita which feeds on privet species in its native range and causes damage to leaves and young shoots. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. -

Motuora Native Species Restoration Plan
Motuora Native Species Restoration Plan JUNE 2007 Motuora Native Species Restoration Plan By Robin Gardner-Gee, Sharen Graham, Richard Griffiths, Melinda Habgood, Shelley Heiss Dunlop and Helen Lindsay MOTUORA RESTORATION SOCIETY (INC) PO Box 100-132, NSMC, Auckland. Foreward Deciding to write a Restoration Plan for Motuora was a huge undertaking for a voluntary group, especially since most of those whose help we needed already had busy lives. The project required surveys on the island to establish what plants and animals were already there, followed by much discussion and the writing of the various sections. These sections then had to be edited to make a unified whole. This document could not have been written without the enthusiasm, knowledge, and commitment of a group of keen environmentalists who put in long hours to produce the Restoration Plan. The Motuora Restoration Society thanks the many people and organizations who have provided information, advice and comment on this document. Particular thanks to: Robin Gardner-Gee for her invertebrate knowledge Sharen Graham for her bird knowledge Richard Griffiths for pulling the document together to present an overview of the whole island ecology Melinda Habgood for her reptile knowledge Shelley Heiss-Dunlop for her plant knowledge Helen Lindsay for her input into the plant section and for co-ordinating the project especially in the beginning Te Ngahere Native Forest Management for supporting this project Department of Conservation staff for support and encouragement. The Motuora Restoration Society thanks you all for your generosity in sharing your learning and experience. Ray Lowe Chairman Motuora Restoration Society i ii Executive Summary Motuora is an 80 hectare island in the Hauraki Gulf to the south of Kawau Island. -

Co-Extinction of Mutualistic Species – an Analysis of Ornithophilous Angiosperms in New Zealand
DEPARTMENT OF BIOLOGICAL AND ENVIRONMENTAL SCIENCES CO-EXTINCTION OF MUTUALISTIC SPECIES An analysis of ornithophilous angiosperms in New Zealand Sandra Palmqvist Degree project for Master of Science (120 hec) with a major in Environmental Science ES2500 Examination Course in Environmental Science, 30 hec Second cycle Semester/year: Spring 2021 Supervisor: Søren Faurby - Department of Biological & Environmental Sciences Examiner: Johan Uddling - Department of Biological & Environmental Sciences “Tui. Adult feeding on flax nectar, showing pollen rubbing onto forehead. Dunedin, December 2008. Image © Craig McKenzie by Craig McKenzie.” http://nzbirdsonline.org.nz/sites/all/files/1200543Tui2.jpg Table of Contents Abstract: Co-extinction of mutualistic species – An analysis of ornithophilous angiosperms in New Zealand ..................................................................................................... 1 Populärvetenskaplig sammanfattning: Samutrotning av mutualistiska arter – En analys av fågelpollinerade angiospermer i New Zealand ................................................................... 3 1. Introduction ............................................................................................................................... 5 2. Material and methods ............................................................................................................... 7 2.1 List of plant species, flower colours and conservation status ....................................... 7 2.1.1 Flower Colours .............................................................................................................