October, 2004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
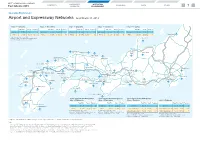
7. Airport and Expressway Networks (PDF, 352KB)
WEST JAPAN RAILWAY COMPANY CORPORATE OPERATING CONTENTS BUSINESS DATA OTHER Fact Sheets 2019 OVERVIEW ENVIRONMENT 7 Operating Environment Airport and Expressway Networks As of March 31, 2019 Tokyo — Fukuoka Tokyo — Hiroshima Tokyo — Okayama Tokyo — Kanazawa Tokyo — Toyama Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Shinkansen 4h 46m 22,950 31 Shinkansen 3h 44m 19,080 46 Shinkansen 3h 09m 17,340 60 Shinkansen 2h 28m 14,120 24 Shinkansen 2h 08m 12,730 24 Niigata Airport Airlines 3h 00m 41,390 54 (19) Airlines 3h 30m 34,890 18 Airlines 3h 10m 33,990 10 Airlines 2h 50m 24,890 10 Airlines 2h 30m 24,890 4 Travel Time and Fare: JAL or ANA Noto Airport Frequency: All airlines. Numbers in parentheses are frequency excluding those of JAL or ANA. Kanazawa Izumo Airport Komatsu Toyama Airport Yonago Airport Airport Tottori Airport Yonago Hagi Iwami Airport Izumo Tajima Airport Gotsu Hamada Tsuruga Yamaguchi Ube Airport Yamaguchi HiroshimaHiroshima Hiroshima Airport Okayama Airport Maibara Kitakyushu Ibaraki Airport Onomichi Hakata KomakiKomaki AirportAirport Okayama KobeKobe ItamiItami AirportAirport Fukuoka Airport Kitakyushu Airport KKurashikiurashiki SSuitauita Iwakuni Kintaikyo NagoyaNagoya Sasebo Tosu Airport Sakaide Shin-OsakaShin-Osaka Tokyo Saga Airport Imabari Kobe Airport Narita Airport Matsuyama Airport Takamatsu Airport Naruto KansaiKansai AirportAirport Haneda Airport Oita Airport Kansai Nagasaki International Airport Chubu International -

Kyushu China Taipei
Sapporo 北京 Japan Seoul South Korea Hiroshima Busan Oita Tokyo Nagoya Fukuoka Osaka Saga Nagasaki Kumamoto Kagoshima Miyazaki Shanghai Kyushu China Taipei Hong Kong Macao Hanoi Taiwan Thailand Vietnam Philippines Bangkok Manila Ho chi minh Malaysia Kuala Lumpur Singapore Singapore Shimonoseki Mojiko Moji Kokura Hakata Takeo Saga Tosu Haiki Onsen Shin Tosu Hita Yufuin Beppu Sasebo Kurume Oita Chikugo Funagoya Chugoku Expressway Huis Ten Bosch Arita Shin Omuta Miyaji Saiki Shin Tamana Aso Kumamoto Higo Ozu Nagasaki Nobeoka Shin Shimonoseki Misumi Yatsushiro Shin Yatsushiro Hitoyoshi Shin Minamata Izumi Yoshimatsu Shimonoseki IC Miyazaki Kirishima Onsen Minami Miyazaki Sendai Kirishima Jingu Aoshima Mojiko IC Hayato Kitago Kagoshima Obi Moji Port Kagoshima Chuo Nichinan Kyushu Expressway Nango Makurazaki Ibusuki Kokura Kitakyushu (Kokura) Shinmonji Port Fukuoka Kokura Higashi IC Kitakyushu Airport Kitakyushu Suou Nada Sea Airport Hakata Port International Terminal Fukuoka IC Hakata Port Hakata Nakatsu Fukuoka Airport Fukuoka Airport Buzen IC Hakata Yobuko Tenjin Usa Beppu Expressway Nakatsu IC Dazaifu IC Oita Airport Nishi Karatsu Oita Airport Kosoku Kiyama Karatsu Saga Tosu JCT Oita Hiji IC Oita AirportRoad Karatsu IC Tosu IC Hirado Imafuku IC Matsuura Shin Tosu Hita IC Oita Expressway Yamashirokubara IC Taniguchi IC Nagasaki Expressway Kurume IC Gulf of Beppu Saga Yamato IC Hita Beppu IC Kurume Hita Yufuin Imari Kurume Saga Yufuin IC Beppu Saza IC Imari Oita Saga Beppu Takeo Onsen Yufuin Oita Sasebo Chikugo Funagoya Amagase Oita IC Nishi-Kyushu -

Aircraft, Defense & Space Domain Business Plan
Aircraft, Defense & Space Business Plan Keisuke Hisakazu Naohiko HIROSE MIZUTANI ABE Senior Vice President, President, Senior Vice President, Head of Commercial Aviation Mitsubishi Aircraft Corporation Head of Integrated Defense Systems & Space Systems June 5, 2018 Mitsubishi Heavy Industries, LTD © MITSUBISHI HEAVY INDUSTRIES, LTD. All Rights Reserved. Contents 1. Business Overview 2. Commercial Aviation Systems Segment 2-1. Overview 2-2. Review of 2015 Medium-Term Business Plan 2-3. Policies and Strategies of 2018 Medium-Term Business Plan 3. MRJ Business 3-1. Development Status 3-2. Preparations for MRJ Production 3-3. Efforts aimed at Commercialization 3-4. MRJ Business Restructuring to Assure Long-Term Business Continuity 4. Integrated Defense & Space Systems Segment 4-1. Overview 4-2. Review of 2015 Medium-Term Business Plan 4-3. Policies and Strategies of 2018 Medium-Term Business Plan © MITSUBISHI HEAVY INDUSTRIES, LTD. All Rights Reserved. 1 1. Business Overview (FY2017 Results and 2018 Business Plan) Integrated Defense & Space Systems Naval ships Aircraft & missile systems Special vehicles Space systems FY2017 FY2020 Commercial Aviation Systems Net sales Net sales Aircraft components ¥722.9 ¥720.0 for Boeing billion billion Aircraft components for Airbus, Bombardier, etc. MRJ Orders Received Net Sales Operating Income / EBIT (in billion yen) 955.0 721.5 0.9 -15.1 -15.0 -45.0 0.0 650.0 700.0 703.4 722.9 700.0 720.0 0.1% 0.0% -2.1% -2.1% -6.4% 2016 2017 2018 2020 2016 2017 2018 2020 2016 2017 2018 2018 2020 (FY) After IFRS adoption © MITSUBISHI HEAVY INDUSTRIES, LTD. -

Collection of Summaries of WAVE Studies and Research (Fiscal 2004) October 2005 Waterfront Vitalization and Environment Research
Collection of summaries of WAVE studies and research (Fiscal 2004) October 2005 Waterfront Vitalization and Environment Research Center Major studies conducted by WAVE are described below. Ⅰ Planning research projects Study Fiscal year when study Study Study outline was conducted Study on Cargo Volume in Main In 1997, the Fourth Tokyo Bay Port Planning Categories at Ports in the Kanto Scheme was drawn up for implementation by 2010. District In March, 2002, the Metropolitan Port Scheme was (Kanto Regional Development formulated as a follow-up to the abovementioned Bureau) scheme. As a next step, it is becoming necessary to consider port planning for the next planning period roughly from 2010 to 2020. In view of these circumstances and on the basis of the content of the information and data collected and collated in fiscal 2003 and the estimation methods considered, in this study, international container cargo volumes at the ports in the Kanto region were estimated. At the same time, information on the trends in domestic unit load cargo was also collected and analyzed, and its demand was estimated. 2004 To be more specific, future volumes of international container cargo and domestic unit load cargo at the international marine container handling ports and domestic unit load handling ports in the Kanto district were estimated by using a model developed according to the data obtained from container cargo flow surveys and net flow surveys. According to this study, the total volume of port cargo in the Kanto district including overseas transshipment is estimated to be 8,200,000 to 9,500,000 TEU in 2015 and 9,200,000 to 10,800,000 TEU in 2020. -

KOD FLYGPLATS AAC Al Arish, Egypt
KOD FLYGPLATS AAC Al Arish, Egypt – Al Arish Airport AAM Mala Mala Airport AAN Al Ain, United Arab Emirates – Al Ain Airport AAQ Anapa Airport – Russia AAT Altay, China – Altay Airport AAX Araxa, Brazil – Araxa Airport ABC Albacete, Spain – Albacete Airport ABE Allentown-Bethlehem-Easton International, PA, USA ABK Kabri Dar, Ethiopia – Kabri Dar Airport ABL Ambler, AK, USA ABM Bamaga, Queensland, Australia ABQ Albuquerque, NM, USA – Albuquerque International A ABR Aberdeen, SD, USA – Aberdeen Regional Airport ABS Abu Simbel, Egypt – Abu Simbel ABT Al-Baha, Saudi Arabia – Al Baha-Al Aqiq Airport ABV Abuja, Nigeria – Abuja International Airport ABX Albury, New South Wales, Australia – Albury ABY Albany, GA, USA – Dougherty County ABZ Aberdeen, Scotland, United Kingdom – Dyce ACA Acapulco, Guerrero, Mexico – Alvarez International ACC Accra, Ghana – Kotoka ACE Lanzarote, Canary Islands, Spain – Lanzarote ACH Altenrhein, Switzerland – Altenrhein Airport ACI Alderney, Channel Islands, United Kingdom – The Bl ACK Nantucket, MA, USA ACT Waco, TX, USA – Madison Cooper ACV Arcata, CA, USA – Arcata/Eureka Airport ACY Atlantic City /Atlantic Cty, NJ, USA – Atlantic Ci ADA Adana, Turkey – Adana ADB Izmir, Turkey – Adnan Menderes ADD Addis Ababa, Ethiopia – Bole ADE Aden, Yemen – Aden International Airport ADJ Amman, Jordan – Civil ADK Adak Island, Alaska, USA, Adak Island Airport ADL Adelaide, South Australia, Australia – Adelaide ADQ Kodiak, AK, USA ADZ San Andres Island, Colombia AED Aleneva, Alaska, USA – Aleneva Airport AEP Buenos Aires, Buenos -

Chapter 2 Aircraft Accident and Serious Incident Investigations
Chapter 2 Aircraft accident and serious incident investigations Chapter 2 Aircraft accident and serious incident investigations 1 Aircraft accidents and serious incidents to be investigated <Aircraft accidents to be investigated> ◎Paragraph 1, Article 2 of the Act for Establishment of the Japan Transport Safety Board(Definition of aircraft accident) The term "Aircraft Accident" as used in this Act shall mean the accident listed in each of the items in paragraph 1 of Article 76 of the Civil Aeronautics Act. ◎Paragraph 1, Article 76 of the Civil Aeronautics Act (Obligation to report) 1 Crash, collision or fire of aircraft; 2 Injury or death of any person, or destruction of any object caused by aircraft; 3 Death (except those specified in Ordinances of the Ministry of Land, Infrastructure, Transport and Tourism) or disappearance of any person on board the aircraft; 4 Contact with other aircraft; and 5 Other accidents relating to aircraft specified in Ordinances of the Ministry of Land, Infrastructure, Transport and Tourism. ◎Article 165-3 of the Ordinance for Enforcement of the Civil Aeronautics Act (Accidents related to aircraft prescribed in the Ordinances of the Ministry of Land, Infrastructure, Transport and Tourism under item 5 of the paragraph1 of the Article 76 of the Act) The cases (excluding cases where the repair of a subject aircraft does not correspond to the major repair work) where navigating aircraft is damaged (except the sole damage of engine, cowling, engine accessory, propeller, wing tip, antenna, tire, brake or fairing). <Aircraft serious incidents to be investigated> ◎Item 2, Paragraph 2, Article 2 of the Act for Establishment of the Japan Transport Safety Board (Definition of aircraft serious incident) A situation where a pilot in command of an aircraft during flight recognized a risk of collision or contact with any other aircraft, or any other situations prescribed by the Ordinances of Ministry of Land, Infrastructure, Transport and Tourism under Article 76-2 of the Civil Aeronautics Act. -

Guide to Importing Dogs and Cats Into Japan from Non-Designated Regions (Final Revision: Mar., 2021)
Guide to importing dogs and cats (from Non-designated regions) Appended Form 5-2 Guide to importing dogs and cats into Japan from Non-designated regions (Final revision: Mar., 2021) Animal Quarantine Service Ministry of Agriculture, Forestry and Fisheries What are the “Non-designated regions”? All countries or regions other than Iceland, Australia, New Zealand, Fiji Islands, Hawaii and Guam. *The designation is subject to change due to the prevalence of rabies or the revision of quarantine regulations. This guide describes the requirements and necessary procedures for importing dogs and cats into Japan from Non-designated regions. We strongly recommend reading this guide if you are planning to bring dogs or cats into Japan. For further information, feel free to contact Animal Quarantine Service. 1 Guide to importing dogs and cats (from Non-designated regions) Contents A. Introduction ........................................................................................................................ 3 Importer’s responsibility................................................................................................... 3 Animals covered by this guide ......................................................................................... 3 B. Import quarantine procedures ............................................................................................ 4 Flow chart .......................................................................................................................... 4 STEP 1: Microchip ............................................................................................................ -

Kyushu,Yamaguchi
World Heritage information facilities Iron Coal World Heritage information facilities Iron Coal Infancy and Steel Shipbuilding Mining Infancy and Steel Shipbuilding Mining ew Photo Local tourism information facilities Local tourism information facilities UNESCO World Heritage Vi s Kitakyushu City, Fukuoka pref./Nakama City, Fukuoka pref. Saga City, Saga pref. YAWATA Shokasonjuku SAGA Academy The first modern integrated iron and steel works in Japan A base for the acquisition and practice of Western shipbuilding techniques AR Map The imperial Steel Works,Japan Mietsu Naval Dock First Head Office 30 minutes by city bus from JR Saga Station Bus Shoin Yoshida Viewing space : 10 minute walk from Space Center, and a five minute walk from Sano Tsunetami Kinen- Kyushu,Yamaguchi ● World Station on the JR Kagoshima Main Line (Take the N 1: 900,000 0 10 20㎞ kan Iriguchi bus stop 30 minutes by Nishitetsu Bus from underground passageway facing the entrance to Space Hagi Iwami Airport Nishitetsu- Yanagawa Station, and a five minute walk from World ) *the inner area isn't open to the public 191 Hayatsue bus stop, the final stop a ©Yawata Works, to c r na ● it v NIPPON STEEL & ● Edamitsu, Yahatahigashi-ku, Kitakyushu-city, Fukuoka Key Component Part Toll Road OazaHayatsuetsu, Kawasoe-town/OazaTameshige, ig SUMITOMO METAL Morodomi-town, Saga-city, Saga m a CORPORATION s t ☎ 093-541-4189 Interchange n i a o City of the Component Part ☎ 0952-40-7105 n Junction Choshu Five r Shimane Prefecture Tsunetami Sano Memorial Museum 0952-34-9455 T [ Not open to the public] -

2016-2017 Unionpay International Global 100 Airport Duty Free Shops Campaign
2016-2017 UnionPay International Global 100 Airport Duty Free Shops Campaign Country/R # # Airport Duty Free Shop Promotion period Offer detail Location egion Arrival Hall, Level 5/Departure Hong Hong Kong From Jul 15, 2016 to Oct 15, 2016; from HKD200 off with a minimum purchase 1 1 1 DFS Hall, level 6, Terminal 1, Hong Kong International Airport Dec 15, 2016 to Feb 15, 2017 over HKD2,000 per single transaction Kong International Airport 1. NTD 1,200 off on one purchase of International Departure/Arrival From April 15, 2016 to May 31, 2016 Taipei Taoyuan NTD 30,000 or above area A, B, Terminal 1, 2 2 Everrich Duty Free From Aug 1, 2016 to Oct 31, 2016 International Airport 2. NTD 4,000 off on one purchase of International Departure/Arrival From Jan 1, 2017 to Feb 28, 2017 NTD 80,000 or above area C, Terminal 2 1. NTD 1,200 off on one purchase of From April 15, 2016 to May 31, 2016 Tasa Meng Duty Free NTD 30,000 or above International Departure/Arrival 3 3 Tasa Meng Duty Free From Aug 1, 2016 to Oct 31, 2016 Shop 2. NTD 4,000 off on one purchase of area D, Terminal 2 From Jan 1, 2017 to Feb 28, 2017 NTD 80,000 or above 1. NTD 1,200 off on one purchase of From April 15, 2016 to May 31, 2016 Taipei Songshan NTD 30,000 or above International Departure/Arrival 4 4 Everrich Duty Free From Aug 1, 2016 to Oct 31, 2016 Airport 2. -

Contents Contents
CONTENTS 6 Preface 54 Heydar Aliyev International Airport 1 0 2 Carrasco International Airport 1 5 2 Ishigaki Airport Passenger Terminal 202 Tokushima Airport Passenger Terminal 250 The Circle at Zurich Airport Arup Rafael Viñoly Architects Azusa Sekkei, Miyahira Sekkei, Azusa Sekkei Riken Yamamoto & Field Shop Sugama Architectural Office Entire Airports 58 Spaceport America 206 Cape Town International Airport 254 Birmingham Airport International Pier Foster+Partner Terminals 1 5 6 Bergen Airport Flesland Terminal 2010 Pascall+Watson architects Narud-Stokke-Wiig Architects & Planners Blueprint Architects 12 Indianapolis International Airport 62 Shenzhen Bao’an International Airport 256 HON Circle Terminal HOK Massimiliano and Doriana Fuksas 1 0 8 San Francisco International Airport 160 Heathrow Airport Terminal 2 210 Los Angeles International Hollin + Radoske Terminal 2 Vidal y Asociados arquitectos, Foster+Partners Fentress Architects 16 Lleida-Alguaire Airport 64 Farnborough Airport Gensler 260 Frankfurt Control Tower b720 Fermín Vázquez Arquitectos 3DReid 164 Mineta San Jose International 2 1 4 Kitakyushu Airport Passenger Terminal Ondra & Partner Architects 112 Madrid-Barajas Airport Terminal 4 Terminal B Asuza Sekkei, HOK 20 Chicago O’Hare International Airport 68 Kutaisi Airport Rogers Stirk Harbour + Partners, Estudio Lamela Fentress Architects 264 Cologne/Bonn Airport Starwalk Murphy/Jahn Architects UNStudio 216 Singapore Changi Airport Terminal 1 1 1 6 Munich Airport Terminal 2 168 Mineta San Jose International Passenger Terminal -

Ministerial Ordinance for Enforcement of the Act on Domestic Animal Infectious Diseases Control (Ministry of Agriculture, Forestry and Fisheries Ordinance No
Ministerial Ordinance for Enforcement of the Act on Domestic Animal Infectious Diseases Control (Ministry of Agriculture, Forestry and Fisheries Ordinance No. 35, 1953) Chapter I General Provisions (Pathogens of piroplasmosis, anaplasmosis and avian salmonellosis) Article 1 The pathogens of piroplasmosis, anaplasmosis, and avian salmonellosis prescribed by the Ordinance of the Ministry of Agriculture, Forestry and Fisheries referred to in the table of Article 2 paragraph 1 of the Act on Domestic Animal Infectious Diseases Control (hereinafter referred to as "the Act") and the table of Article 1 of the Government Ordinance for Enforcement of the Act on Domestic Animal Infectious Diseases Control (Government Ordinance No. 235, 1953; hereafter referred to as "the Ordinance") shall be as listed in the following table: Infectious disease Pathogens Piroplasmosis Babesia bigemina, Babesia bovis, Babesia equi, Babesia caballi, Theileria parva,Theileria annulata Anaplasmosis Anaplasma marginale Avian salmonellosis Salmonella enterica (Only those whose serotype is Salmonella gallinarum and whose biotype is Salmonella pullorum or Salmonella gallinarum) (Highly pathogenic Newcastle disease) Article 1-2 Newcastle disease prescribed by the Ministerial Ordinance referred to in the table of Article 2 paragraph 1 of the Act and the table of Article 1 of the Government Ordinance shall be the following: (1) Newcastle disease, whose ICPI (index indicating pathogenicity of a pathogen obtained in an intracerebral inoculation test; hereinafter referred to as the same) of the pathogen is 0.7 or more in one-day old chicks. (2) Newcastle disease to which both of the following items are applied: a. Of the 113rd to 116th amino acid residues of the F protein of the causative agent, three or more are estimated to be an arginine or a lysine residue. -

Artnerships for the Sustainable Development of Cities in the APEC
7. Kitakyushu City, Japan Hitomi Nakanishi and Hisashi Shibata 7.1 INTRODUCTION The City of Kitakyushu in Fukuoka Prefecture is the 13th largest city in Japan. Located on Kyushu island just south of the Japanese main island, it is regarded as a gateway to Asian economies. The city was developed by the steel industry in the modern era (1900s), and grew to become one of the largest industrial zones in Japan. However, by the 1950s and 1960s, its rapid development had led to air and water pollution (Photo 7.2). The Dokai Bay area was contaminated by factory emissions and industrial and domestic wastewater, and came to be dubbed the ‘sea of death’.322 The local administration was forced to act, and the city dramatically recovered from the environmental degradation. Kitakyushu set out to become the World Capital of Sustainable Development; and it became known for its sustainability initiatives, many of which involved partnerships with residents, enterprises, research institutes and government administrations. This chapter illustrates Kitakyushu’s concept of urban management, exemplified by the Energetic Kitakyushu Plan.323 Secondary data sourced from the City of Kitakyushu and academic articles were drawn upon for this case study. Photo 7.1 City of Kitakyushu Source: City of Kitakyushu 183 Photo 7.2 Overcoming Severe Environmental Pollution, City of Kitakyushu In the 1950s & 1960s Present Credit: City of Kitakyushu. The chapter discusses some of the measures implemented by Kitakyushu City in moving from a ‘grey city’ to a green and a sustainable city. First, Kitakyushu’s economic dynamics, its infrastructure, and its social, environmental and governance systems are described in detail.