Astereae Compositae) and Other Chrysothamnus Species Using Paper Chromatography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eriodictyon Trichocalyx A
I. SPECIES Eriodictyon trichocalyx A. Heller NRCS CODE: Family: Boraginaceae ERTR7 (formerly placed in Hydrophyllaceae) Order: Solanales Subclass: Asteridae Class: Magnoliopsida juvenile plant, August 2010 A. Montalvo , 2010, San Bernardino Co. E. t. var. trichocalyx A. Subspecific taxa ERTRT4 1. E. trichocalyx var. trichocalyx ERTRL2 2. E. trichocalyx var. lanatum (Brand) Jeps. B. Synonyms 1. E. angustifolium var. pubens Gray; E. californicum var. pubens Brand (Abrams & Smiley 1915) 2. E. lanatum (Brand) Abrams; E. trichocalyx A. Heller ssp. lanatum (Brand) Munz; E. californicum. Greene var. lanatum Brand; E. californicum subsp. australe var. lanatum Brand (Abrams & Smiley 1915) C.Common name 1. hairy yerba santa (Roberts et al. 2004; USDA Plants; Jepson eFlora 2015); shiny-leaf yerba santa (Rebman & Simpson 2006); 2. San Diego yerba santa (McMinn 1939, Jepson eFlora 2015); hairy yerba santa (Rebman & Simpson 2006) D.Taxonomic relationships Plants are in the subfamily Hydrophylloideae of the Boraginaceae along with the genera Phacelia, Hydrophyllum, Nemophila, Nama, Emmenanthe, and Eucrypta, all of which are herbaceous and occur in the western US and California. The genus Nama has been identified as a close relative to Eriodictyon (Ferguson 1999). Eriodictyon, Nama, and Turricula, have recently been placed in the new family Namaceae (Luebert et al. 2016). E.Related taxa in region Hannan (2013) recognizes 10 species of Eriodictyon in California, six of which have subspecific taxa. All but two taxa have occurrences in southern California. Of the southern California taxa, the most closely related taxon based on DNA sequence data is E. crassifolium (Ferguson 1999). There are no morphologically similar species that overlap in distribution with E. -

Plant Guide for Yellow Rabbitbrush (Chrysothamnus Viscidiflorus)
Plant Guide valuable forage especially during late fall and early winter YELLOW after more desirable forage has been utilized (Tirmenstein, 1999). Palatability and usage vary between RABBITBRUSH subspecies of yellow rabbitbrush (McArthuer et al., 1979). Chrysothamnus viscidiflorus (Hook.) Nutt. Yellow rabbitbrush provides cover and nesting habitat for Plant Symbol = CHVI8 sage-grouse, small birds and rodents (Gregg et al., 1994). Black-tailed jackrabbits consume large quantities of Contributed by: USDA NRCS Idaho Plant Materials yellow rabbitbrush during winter and early spring when Program plants are dormant (Curie and Goodwin, 1966). Yellow rabbitbrush provides late summer and fall forage for butterflies. Unpublished field reports indicate visitation from bordered patch butterflies (Chlosyne lacinia), Mormon metalmark (Apodemia mormo), mourning cloak (Nymphalis antiopa), common checkered skipper (Pyrgus communis), and Weidemeyer’s admiral (Limenitis weidemeyerii). Restoration: Yellow rabbitbrush is a seral species which colonizes disturbed areas making it well suited for use in restoration and revegetation plantings. It can be established from direct seeding and will spread via windborne seed. It has been successfully used for revegetating depleted rangelands, strip mines and roadsides (Plummer, 1977). Status Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g., threatened or endangered species, state noxious status, and wetland indicator values). Description General: Sunflower family (Asteraceae). Yellow rabbitbrush is a low- to moderate-growing shrub reaching mature heights of 20 to 100 cm (8 to 39 in) tall. The stems Al Schneider @ USDA-NRCS PLANTS Database can be glabrous or pubescent depending on variety, and are covered with pale green to white-gray bark. -

A Checklist of the Vascular Flora of the Mary K. Oxley Nature Center, Tulsa County, Oklahoma
Oklahoma Native Plant Record 29 Volume 13, December 2013 A CHECKLIST OF THE VASCULAR FLORA OF THE MARY K. OXLEY NATURE CENTER, TULSA COUNTY, OKLAHOMA Amy K. Buthod Oklahoma Biological Survey Oklahoma Natural Heritage Inventory Robert Bebb Herbarium University of Oklahoma Norman, OK 73019-0575 (405) 325-4034 Email: [email protected] Keywords: flora, exotics, inventory ABSTRACT This paper reports the results of an inventory of the vascular flora of the Mary K. Oxley Nature Center in Tulsa, Oklahoma. A total of 342 taxa from 75 families and 237 genera were collected from four main vegetation types. The families Asteraceae and Poaceae were the largest, with 49 and 42 taxa, respectively. Fifty-eight exotic taxa were found, representing 17% of the total flora. Twelve taxa tracked by the Oklahoma Natural Heritage Inventory were present. INTRODUCTION clayey sediment (USDA Soil Conservation Service 1977). Climate is Subtropical The objective of this study was to Humid, and summers are humid and warm inventory the vascular plants of the Mary K. with a mean July temperature of 27.5° C Oxley Nature Center (ONC) and to prepare (81.5° F). Winters are mild and short with a a list and voucher specimens for Oxley mean January temperature of 1.5° C personnel to use in education and outreach. (34.7° F) (Trewartha 1968). Mean annual Located within the 1,165.0 ha (2878 ac) precipitation is 106.5 cm (41.929 in), with Mohawk Park in northwestern Tulsa most occurring in the spring and fall County (ONC headquarters located at (Oklahoma Climatological Survey 2013). -

Indian Joe Springs Ecological Reserve Land Management Plan (LMP)
State of California California Natural Resources Agency DEPARTMENT OF FISH AND WILDLIFE FINAL LAND MANAGEMENT PLAN for INDIAN JOE SPRINGS ECOLOGICAL RESERVE Inyo County, California April, 2018 Indian Joe Springs Ecological Reserve -1- April, 2018 Land Management Plan INDIAN JOE SPRINGS ECOLOGICAL RESERVE FINAL LAND MANAGEMENT PLAN Indian Joe Springs Ecological Reserve -ii- April, 2018 Land Management Plan This Page Intentionally Left Blank Indian Joe Springs Ecological Reserve -iv- April, 2018 Land Management Plan TABLE OF CONTENTS Page No. TABLE OF CONTENTS v LIST OF FIGURES vii LIST OF TABLES vii I. INTRODUCTION 1 A. Purpose of and History of Acquisition 1 B. Purpose of This Management Plan 1 II. PROPERTY DESCRIPTION 2 A. Geographical Setting 2 B. Property Boundaries and Adjacent Lands 2 C. Geology, Soils, Climate, Hydrology 3 D. Cultural Features 13 III. HABITAT AND SPECIES DESCRIPTION 15 A. Vegetation Communities, Habitats 15 B. Plant Species 18 C. Animal Species 20 D. Threatened, Rare or Endangered Species 22 IV. MANAGEMENT GOALS AND ENVIRONMENTAL IMPACTS 35 A. Definition of Terms Used in This Plan 35 B. Biological Elements: Goals & Environmental Impacts 35 C. Biological Monitoring Element: Goals & Environmental Impacts 39 D. Public Use Elements: Goals & Environmental Impacts 41 E. Facility Maintenance Elements: Goals & Environmental Impacts 44 F. Cultural Resource Elements: Goals & Environmental Impacts 46 G. Administrative Elements: Goals & Environmental Impacts 46 V. OPERATIONS AND MAINTENANCE SUMMARY 48 Existing Staff and Additional Personnel Needs Summary 48 VI. CLIMATE CHANGE STRATEGIES 48 VII. FUTURE REVISIONS TO LAND MANAGEMENT PLANS 51 VIII. REFERENCES 54 Indian Joe Springs Ecological Reserve -v- April, 2018 Land Management Plan APPENDICES: A. -

US Fish and Wildlife Service
BARNEBY REED-MUSTARD (S. barnebyi ) CLAY REED-MUSTARD SHRUBBY REED-MUSTARD (S,arguillacea) (S. suffrutescens) .-~ U.S. Fish and Wildlife Service UTAH REED—MUSTARDS: CLAY REED-MUSTARD (SCHOENOCRAMBE ARGILLACEA) BARNEBY REED—MUSTARD (SCHOENOCRAMBE BARNEBYI) SI-IRUBBY REED-MUSTARD (SCHOENOCRAMBE SUFFRUTESCENS) RECOVERY PLAN Prepared by Region 6, U.S. Fish and Wildlife Service Approved: Date: (~19~- Recovery plans delineate reasonable actions which are believed to be required to recover and/or protect the species. Plans are prepared by the U.S. Fish and Wildlife Service, sometimes with the assistance of recovery teams, contractors, State agencies, and others. Objectives will only be attained and funds expended contingent upon appropriations, priorities, and other budgetary constraints. Recovery plans do not necessarily represent the views or the official positions or approvals of any individuals or agencies, other than the U.S. Fish and Wildlife Service, involved in the plan formulation. They represent the official position of the U.S. Fish and Wildlife Service only after they have been signed by the Regional Director or Director as an~roved Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. Literature Citation should read as follows: U.S. Fish and Wildlife Service. 1994. Utah reed—mustards: clay reed—mustard (Schoenocrambe argillacea), Barneby reed-mustard (Schoenocrambe barnebyl), shrubby reed—mustard (Schoenacranibe suffrutescens) recovery plan. Denver, Colorado. 22 pp. Additional copies may be purchased from: Fish and Wildlife Reference Service 5430 Grosvenor Lane, Suite 110 Bethesda, Maryland 20814 Telephone: 301/492—6403 or 1—800—582—3421 The fee for the plan varies depending on the number of pages of the plan. -

Infection in Kangaroo Rats (Dipodomys Spp.): Effects on Digestive Efficiency
Great Basin Naturalist Volume 55 Number 1 Article 8 1-16-1995 Whipworm (Trichuris dipodomys) infection in kangaroo rats (Dipodomys spp.): effects on digestive efficiency James C. Munger Boise State University, Boise, idaho Todd A. Slichter Boise State University, Boise, Idaho Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Munger, James C. and Slichter, Todd A. (1995) "Whipworm (Trichuris dipodomys) infection in kangaroo rats (Dipodomys spp.): effects on digestive efficiency," Great Basin Naturalist: Vol. 55 : No. 1 , Article 8. Available at: https://scholarsarchive.byu.edu/gbn/vol55/iss1/8 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Great Basin Naturalist 55(1), © 1995, pp. 74-77 WHIPWORM (TRICHURIS DIPODOMYS) INFECTION IN KANGAROO RATS (DIPODOMYS SPP): EFFECTS ON DIGESTIVE EFFICIENCY James C. Mungerl and Todd A. Slichterl ABSTRACT.-To dcterminc whether infections by whipworms (Trichuris dipodumys [Nematoda: Trichurata: Trichuridae]) might affect digestive eHlciency and therefore enel"/,,'Y budgets of two species ofkangaroo rats (Dipodomys microps and Dipodumys urdU [Rodentia: Heteromyidae]), we compared the apparent dry matter digestibility of three groups of hosts: those naturally infected with whipworms, those naturally uninfected with whipworms, and those origi~ nally naturally infected but later deinfected by treatment with the anthelminthic Ivermectin. Prevalence of T. dipodomys was higher in D. rnicrops (.53%) than in D. ordU (14%). Apparent dry matter digestibility was reduced by whipworm infection in D. -

Notes on Variation and Geography in Rayjacksonia Phyllocephala (Asteraceae: Astereae)
Nesom, G.L., D.J. Rosen, and S.K. Lawrence. 2013. Notes on variation and geography in Rayjacksonia phyllocephala (Asteraceae: Astereae). Phytoneuron 2013-53: 1–15. Published 12 August 2013. ISSN 2153 733X NOTES ON VARIATION AND GEOGRAPHY IN RAYJACKSONIA PHYLLOCEPHALA (ASTERACEAE: ASTEREAE) GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 [email protected] DAVID J. ROSEN Department of Biology Lee College Baytown, Texas 77522-0818 [email protected] SHIRON K. LAWRENCE Department of Biology Lee College Baytown, Texas 77522-0818 ABSTRACT Inflorescences of Rayjacksonia phyllocephala in the disjunct Florida population system are characterized by heads on peduncles with leaves mostly reduced to linear bracts; heads in inflorescences of the Mexico-Texas-Louisiana system are immediately subtended by relatively unreduced leaves. The difference is consistent and justifies recognition of the Florida system as R. phyllocephala var. megacephala (Nash) D.B. Ward. Scattered waifs between the two systems are identified here as one or the other variety, directly implying their area of origin. In the eastern range of var. phyllocephala , at least from Brazoria County, Texas, eastward about 300 miles to central Louisiana, leaf margins vary from entire to deeply toothed-spinulose. In contrast, margins are invariably toothed-spinulose in var. megacephala as well as in the rest of southeastern Texas (from Brazoria County southwest) into Tamaulipas, Mexico. In some of the populations with variable leaf margins, 70-95% of the individuals have entire to mostly entire margins. KEY WORDS : Rayjacksonia , morphological variation, leaf margins, disjunction, waifs Rayjacksonia phyllocephala (DC.) Hartman & Lane (Gulf Coast camphor-daisy) is an abundant and conspicuous species of the shore vegetation around the Gulf of Mexico. -

Literature Cited
Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volumes 19, 20, and 21, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbre- viated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu and E. A. Mennega (1992+). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix “a”; thus, the sequence of explicit suffixes begins with “b”. Works missing from any suffixed sequence here are ones cited elsewhere in the Flora that are not pertinent in these volumes. -

Phytologia an International Journal to Expedite
PHYTOLOGIA An intern ational jou rnal to ex edite la n t s stematic b to eo ra bi l p p y , p y g g p ca and ecological pu blication 1 S e ember 1 1 Vo l. 7 pt 99 CONTENTS TRE M N w ll n n n t r c l fl r . A ne CUA CASAS . c o o o o o o , J , is e a e us tes e pi a a XX species of Humiriastru m 1 65 R T F w f S . rm l rr t n f t c th in the th rn OSS . o co c o o o c t o , , a e i spe i i epi e s s u e . N N H N A eratina nd w f B artlettina ROB S O . o t on a ne c o / I , , es g a spe ies (Eupato rieae : Asteraceae) 1 7 1 /R )BIN N H N w w f C SO . e c and ne comb n on o Crit oniinae from , , spe ies i ati s Meso ame ri ca (Eupato rieae : Asteraceae) 1 76 ’ R B N N H Tw w f Fleiscbman m a fr m M r O S O . o ne c o o m c / I , , spe ies o es a e i a (Eupatorieae : As t eraceae) 1 8 1 /ROBIN N Tw w M le a M SO H. o ne c o f i an i in m r c m , , spe ies esoa e i a (Eupatorieae : As te raceae) 1 84 E AL N D L f f a a r i S C O A F . -

The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014
The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014 In the pages that follow are treatments that have been revised since the publication of the Jepson eFlora, Revision 1 (July 2013). The information in these revisions is intended to supersede that in the second edition of The Jepson Manual (2012). The revised treatments, as well as errata and other small changes not noted here, are included in the Jepson eFlora (http://ucjeps.berkeley.edu/IJM.html). For a list of errata and small changes in treatments that are not included here, please see: http://ucjeps.berkeley.edu/JM12_errata.html Citation for the entire Jepson eFlora: Jepson Flora Project (eds.) [year] Jepson eFlora, http://ucjeps.berkeley.edu/IJM.html [accessed on month, day, year] Citation for an individual treatment in this supplement: [Author of taxon treatment] 2014. [Taxon name], Revision 2, in Jepson Flora Project (eds.) Jepson eFlora, [URL for treatment]. Accessed on [month, day, year]. Copyright © 2014 Regents of the University of California Supplement II, Page 1 Summary of changes made in Revision 2 of the Jepson eFlora, December 2014 PTERIDACEAE *Pteridaceae key to genera: All of the CA members of Cheilanthes transferred to Myriopteris *Cheilanthes: Cheilanthes clevelandii D. C. Eaton changed to Myriopteris clevelandii (D. C. Eaton) Grusz & Windham, as native Cheilanthes cooperae D. C. Eaton changed to Myriopteris cooperae (D. C. Eaton) Grusz & Windham, as native Cheilanthes covillei Maxon changed to Myriopteris covillei (Maxon) Á. Löve & D. Löve, as native Cheilanthes feei T. Moore changed to Myriopteris gracilis Fée, as native Cheilanthes gracillima D. -

Flora of the Stansbury Mountains, Utah
Great Basin Naturalist Volume 43 Number 4 Article 11 10-31-1983 Flora of the Stansbury Mountains, Utah Alan C. Taye U.S. Army Intelligence Center and School, Fort Huachuca, Arizona Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Taye, Alan C. (1983) "Flora of the Stansbury Mountains, Utah," Great Basin Naturalist: Vol. 43 : No. 4 , Article 11. Available at: https://scholarsarchive.byu.edu/gbn/vol43/iss4/11 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. FLORA OF THE STANSBURY MOUNTAINS, UTAH Alan C. Taye' Abstract.— The Stansbury Mountains of north central Utah rise over 2000 m above surrounding desert valleys to a maximum elevation of 3362 m on Deseret Peak. Because of the great variety of environmental conditions that can be found in the Stansburys, a wide range of plant species and vegetation types (from shadscale desert to alpine mead- ow) exist there. This paper presents an annotated list of 594 vascular plant species in 315 genera and 78 families. The largest families are Asteraceae (98 species), Poaceae (71), Brassicaceae (33), Fabaceae (27), and Rosaceae (26). Elymiis flcwescens was previously unreported from Utah. Statistical comparison of the Stansbury flora with neighboring mountain floras indicates that the Wasatch Mountains lying 65 km to the east have probably been the primary source area for development of the Stansbury flora. -
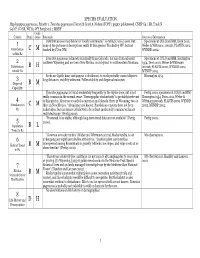
1 C M 2 B H 3 B M 4 C M 5 B L 6 B M 7 B M 8
SPECIES EVALUATION Haplopappus pygmaeus, Priority 1. Tonestus pygmaeus (Torrey & Gray) A. Nelson (TOPY). pygmy goldenweed. CNHP G4 / SR, Track N G4 N?. CO SR, WY S1. WY Peripheral 2 MBNF Confi- Criteria Rank dence Rationale Sources of Information Distribution (see map below) is “nearly continuous,” so rating C was chosen, but Specimens at COLO and RM, Dorn 2001, 1 none of the pictures or descriptions really fit this species. Tracked by WY, but not Weber & Wittmann 2001ab, PLANTS 2002, Distribution C M tracked by CO or NM. WYNDD 2002. within R2 Tonestus pygmaeus is known principally from Colorado, but also from adjacent Specimens at COLO and RM, Harrington 2 southern Wyoming and northern New Mexico, and disjunct in southwestern Montana. 1954, Dorn 2001, Weber & Wittmann Distribution B H 2001ab, PLANTS 2002, WYNDD 2002, outside R2 MTNHP 2002. Seeds are lightly hairy and pappus is deciduous, so seeds probably cannot disperse Harrington 1954. 3 long distances; viability unknown. Pollen viability and dispersal unknown. Dispersal B M Capability Tonestus pygmaeus is found moderately frequently in the Alpine zone, but is not Fertig 2000, specimens at COLO and RM, really common in the normal sense. “Demographic stochasticity” is probably irrelevant Harrington 1954, Dorn 2001, Weber & 4 to this species. About 50 recorded occurrences in Colorado, three in Wyoming, two or Wittmann 2001ab, PLANTS 2002, WYNDD Abundance in C M three in New Mexico. “Abundance not known. Population censuses have not been 2002, MTNHP 2002. R2 undertaken, but the species is believed to be at least moderately common within its restricted range” (Fertig 2000).