Comparative Molecular Portraits of Human Unfertilized Oocytes And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Get High-Res Image
50 40 30 20 10 226 155 243 73 63 51 # mutations Non syn. Syn. *CpG->T *Cp(A/C/T)->T A->G transver indel+null double_null # mutations/"< 0 10 15 20 25 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 0% 0% 1% 2% 1% 1% 1% 1% 0% 1% 1% 4% 1% 1% 1% 2% 0% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 2% 1% 1% 1% 1% 1% 0% 1% 1% 1% 1% 2% 1% 1% 3% 1% 1% 1% 1% 2% 1% 1% 1% 2% 1% 1% 2% 1% 1% 1% 1% 1% 2% 1% 1% 1% 1% 1% 2% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 2% 1% 1% 5% 1% 1% 1% 2% 1% 1% 1% 1% 1% 2% 1% 1% 1% 1% 1% 1% 2% 2% 1% 2% 2% 13% 2% 4% 4% 9% 4% 19% 0% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 1% 2% 2% 2% 2% 2% 2% 2% 2% 2% 2% 2% 2% 2% 2% 2% 3% 3% 3% 4% 5% 29% 0 5 %IRC-A$'3778'TP %IRC-C3'5677-TP%IRC'(0'4710'TP%IRC-A3'3383'TP %IRC-E.'5907-TP%IRC-A3'3382'TP%IRC'(0'4712'TP %IRC-A3'3372'TP%IRC-A3'3316'TP%IRC-A%'3444'TP %IRC-C*'59,2-TP%IRC-A3'3378'TP%IRC-A3'3308'TP %IRC'(P'5184'TP%IRC'(0'5693'TP%IRC'(,'5159'TP %IRC'(P'4968'TP%IRC'(P'5196'TP%IRC-C3'5675-TP %IRC-C--55,0-TP%IRC'(P'5169'TP%IRC'(0'5699'TP %IRC-C3'5676-TP%IRC'(P'5198'TP%IRC-C3'56,4-TP %IRC-A3'3331'TP%IRC'(0'5399'TP%IRC-C*'5470-TP %IRC'(0'4706'TP%IRC'(0'4,16'TP%IRC'(P'4159'TP %IRC'(P'5183'TP%IRC'(0'5703'TP%IRC'(P'5187'TP %IRC'(,'4148'TP%IRC-C*'5460-TP%IRC'(P'4164'TP %IRC'(0'5085'TP%IRC-C3'4903-TP%IRC-C3'4634-TP %IRC'(4'5,32'TP%IRC'(4'5,36'TP%IRC'(P'49,,'TP %IRC'(P'4960'TP%IRC'(0'5696'TP%IRC-C3'5679-TP %IRC'(0'5115'TP%IRC'(P'4989'TP%IRC-C*'5465-TP %IRC'(P'4999'TP%IRC'(P'5181'TP%IRC'(P'5200'TP %IRC'(P'4964'TP%IRC'(0'5705'TP%IRC'(,'4154'TP %IRC-C3'4920-TP%IRC-C*'5466-TP%IRC-C3'6030-TP -

The Hypothalamus As a Hub for SARS-Cov-2 Brain Infection and Pathogenesis
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.08.139329; this version posted June 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis Sreekala Nampoothiri1,2#, Florent Sauve1,2#, Gaëtan Ternier1,2ƒ, Daniela Fernandois1,2 ƒ, Caio Coelho1,2, Monica ImBernon1,2, Eleonora Deligia1,2, Romain PerBet1, Vincent Florent1,2,3, Marc Baroncini1,2, Florence Pasquier1,4, François Trottein5, Claude-Alain Maurage1,2, Virginie Mattot1,2‡, Paolo GiacoBini1,2‡, S. Rasika1,2‡*, Vincent Prevot1,2‡* 1 Univ. Lille, Inserm, CHU Lille, Lille Neuroscience & Cognition, DistAlz, UMR-S 1172, Lille, France 2 LaBoratorY of Development and PlasticitY of the Neuroendocrine Brain, FHU 1000 daYs for health, EGID, School of Medicine, Lille, France 3 Nutrition, Arras General Hospital, Arras, France 4 Centre mémoire ressources et recherche, CHU Lille, LiCEND, Lille, France 5 Univ. Lille, CNRS, INSERM, CHU Lille, Institut Pasteur de Lille, U1019 - UMR 8204 - CIIL - Center for Infection and ImmunitY of Lille (CIIL), Lille, France. # and ƒ These authors contriButed equallY to this work. ‡ These authors directed this work *Correspondence to: [email protected] and [email protected] Short title: Covid-19: the hypothalamic hypothesis 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.06.08.139329; this version posted June 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Amino Acid Sequences Directed Against Cxcr4 And
(19) TZZ ¥¥_T (11) EP 2 285 833 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07K 16/28 (2006.01) A61K 39/395 (2006.01) 17.12.2014 Bulletin 2014/51 A61P 31/18 (2006.01) A61P 35/00 (2006.01) (21) Application number: 09745851.7 (86) International application number: PCT/EP2009/056026 (22) Date of filing: 18.05.2009 (87) International publication number: WO 2009/138519 (19.11.2009 Gazette 2009/47) (54) AMINO ACID SEQUENCES DIRECTED AGAINST CXCR4 AND OTHER GPCRs AND COMPOUNDS COMPRISING THE SAME GEGEN CXCR4 UND ANDERE GPCR GERICHTETE AMINOSÄURESEQUENZEN SOWIE VERBINDUNGEN DAMIT SÉQUENCES D’ACIDES AMINÉS DIRIGÉES CONTRE CXCR4 ET AUTRES GPCR ET COMPOSÉS RENFERMANT CES DERNIÈRES (84) Designated Contracting States: (74) Representative: Hoffmann Eitle AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Patent- und Rechtsanwälte PartmbB HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL Arabellastraße 30 PT RO SE SI SK TR 81925 München (DE) (30) Priority: 16.05.2008 US 53847 P (56) References cited: 02.10.2008 US 102142 P EP-A- 1 316 801 WO-A-99/50461 WO-A-03/050531 WO-A-03/066830 (43) Date of publication of application: WO-A-2006/089141 WO-A-2007/051063 23.02.2011 Bulletin 2011/08 • VADAY GAYLE G ET AL: "CXCR4 and CXCL12 (73) Proprietor: Ablynx N.V. (SDF-1) in prostate cancer: inhibitory effects of 9052 Ghent-Zwijnaarde (BE) human single chain Fv antibodies" CLINICAL CANCER RESEARCH, THE AMERICAN (72) Inventors: ASSOCIATION FOR CANCER RESEARCH, US, • BLANCHETOT, Christophe vol.10, no. -

Mean Depth 1000 More Than100×
A B ×20 ×50 ×100 100% 1000 800 90% 600 80% 400 Mean depth 70% 200 0 60% 50% Mean coverage 40% PBL002_T PBL003_T PBL004_T PBL005_T PBL006_T PBL007_T PBL008_T PBL009_T PBL010_T PBL001_P 30% 20% 10% 0% PBL002_T PBL003_T PBL004_T PBL005_T PBL006_T PBL007_T PBL008_T PBL009_T PBL010_T PBL001_P Figure S1. Depths and coverages of targeted deep sequencing. A. Depths of targeted deep sequencing on 10 PBL cases (mean depth = 677). B. Coverages of the targeted sequences analyzed at the indicated depths; 98.8% of the targeted regions were sequenced at a depth more than 100×. A B ×2 ×10 ×20 250 100% 200 90% 150 80% 100 Mean depth 50 70% 0 60% 50% PBL002_T PBL004_T PBL007_T PBL008_T PBL010_T PBL001_P PBL001_N PBL002_N PBL004_N PBL007_N PBL008_N PBL010_N PBL001_M1 PBL001_M2 PBL001_M3 PBL001_M4 PBL001_M5 PBL001_M6 Mean coverage 40% 30% 20% 10% 0% PBL002_T PBL004_T PBL007_T PBL008_T PBL010_T PBL001_P PBL001_N PBL002_N PBL004_N PBL007_N PBL008_N PBL010_N PBL001_M1 PBL001_M2 PBL001_M3 PBL001_M4 PBL001_M5 PBL001_M6 C D 14 80 12 70 10 60 50 8 40 6 30 4 Number of mutations Number of mutations 20 2 10 0 0 PBL002_T PBL004_T PBL007_T PBL008_T PBL010_T PBL001_P PBL001_P PBL001_M1 PBL001_M2 PBL001_M3 PBL001_M4 PBL001_M5 PBL001_M6 Nonsynonymous SNV Stopgain SNV Nonframeshift indels Frameshift indels Splice site Figure S2. Sequencing coverages and numbers of mutations detected by WES. A. Depths of WES on tumor and normal DNA samples from 6 cases (mean depth = 124 for tumor samples and 141 for normal samples). B. Coverages of the exome sequences; 97.5% of the exome sequences were analyzed at a depth more than 20×. C-D. -

Supplementary Data
SUPPLEMENTARY METHODS 1) Characterisation of OCCC cell line gene expression profiles using Prediction Analysis for Microarrays (PAM) The ovarian cancer dataset from Hendrix et al (25) was used to predict the phenotypes of the cell lines used in this study. Hendrix et al (25) analysed a series of 103 ovarian samples using the Affymetrix U133A array platform (GEO: GSE6008). This dataset comprises clear cell (n=8), endometrioid (n=37), mucinous (n=13) and serous epithelial (n=41) primary ovarian carcinomas and samples from 4 normal ovaries. To build the predictor, the Prediction Analysis of Microarrays (PAM) package in R environment was employed (http://rss.acs.unt.edu/Rdoc/library/pamr/html/00Index.html). When more than one probe described the expression of a given gene, we used the probe with the highest median absolute deviation across the samples. The dataset from Hendrix et al. (25) and the dataset of OCCC cell lines described in this manuscript were then overlaid on the basis of 11536 common unique HGNC gene symbols. Only the 99 primary ovarian cancers samples and the four normal ovary samples were used to build the predictor. Following leave one out cross-validation, a predictor based upon 126 genes was able to identify correctly the four distinct phenotypes of primary ovarian tumour samples with a misclassification rate of 18.3%. This predictor was subsequently applied to the expression data from the 12 OCCC cell lines to determine the likeliest phenotype of the OCCC cell lines compared to primary ovarian cancers. Posterior probabilities were estimated for each cell line in comparison to the following phenotypes: clear cell, endometrioid, mucinous and serous epithelial. -

Us 2018 / 0305689 A1
US 20180305689A1 ( 19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 /0305689 A1 Sætrom et al. ( 43 ) Pub . Date: Oct. 25 , 2018 ( 54 ) SARNA COMPOSITIONS AND METHODS OF plication No . 62 /150 , 895 , filed on Apr. 22 , 2015 , USE provisional application No . 62/ 150 ,904 , filed on Apr. 22 , 2015 , provisional application No. 62 / 150 , 908 , (71 ) Applicant: MINA THERAPEUTICS LIMITED , filed on Apr. 22 , 2015 , provisional application No. LONDON (GB ) 62 / 150 , 900 , filed on Apr. 22 , 2015 . (72 ) Inventors : Pål Sætrom , Trondheim (NO ) ; Endre Publication Classification Bakken Stovner , Trondheim (NO ) (51 ) Int . CI. C12N 15 / 113 (2006 .01 ) (21 ) Appl. No. : 15 /568 , 046 (52 ) U . S . CI. (22 ) PCT Filed : Apr. 21 , 2016 CPC .. .. .. C12N 15 / 113 ( 2013 .01 ) ; C12N 2310 / 34 ( 2013. 01 ) ; C12N 2310 /14 (2013 . 01 ) ; C12N ( 86 ) PCT No .: PCT/ GB2016 /051116 2310 / 11 (2013 .01 ) $ 371 ( c ) ( 1 ) , ( 2 ) Date : Oct . 20 , 2017 (57 ) ABSTRACT The invention relates to oligonucleotides , e . g . , saRNAS Related U . S . Application Data useful in upregulating the expression of a target gene and (60 ) Provisional application No . 62 / 150 ,892 , filed on Apr. therapeutic compositions comprising such oligonucleotides . 22 , 2015 , provisional application No . 62 / 150 ,893 , Methods of using the oligonucleotides and the therapeutic filed on Apr. 22 , 2015 , provisional application No . compositions are also provided . 62 / 150 ,897 , filed on Apr. 22 , 2015 , provisional ap Specification includes a Sequence Listing . SARNA sense strand (Fessenger 3 ' SARNA antisense strand (Guide ) Mathew, Si Target antisense RNA transcript, e . g . NAT Target Coding strand Gene Transcription start site ( T55 ) TY{ { ? ? Targeted Target transcript , e . -

Comprehensive Genomic Analysis of Dietary Habits in UK Biobank Identifies Hundreds of Genetic Associations
ARTICLE https://doi.org/10.1038/s41467-020-15193-0 OPEN Comprehensive genomic analysis of dietary habits in UK Biobank identifies hundreds of genetic associations ✉ Joanne B. Cole 1,2,3, Jose C. Florez1,2,4 & Joel N. Hirschhorn1,3,5 Unhealthful dietary habits are leading risk factors for life-altering diseases and mortality. Large-scale biobanks now enable genetic analysis of traits with modest heritability, such as 1234567890():,; diet. We perform a genomewide association on 85 single food intake and 85 principal component-derived dietary patterns from food frequency questionnaires in UK Biobank. We identify 814 associated loci, including olfactory receptor associations with fruit and tea intake; 136 associations are only identified using dietary patterns. Mendelian randomization suggests our top healthful dietary pattern driven by wholemeal vs. white bread consumption is causally influenced by factors correlated with education but is not strongly causal for coronary artery disease or type 2 diabetes. Overall, we demonstrate the value in complementary phenotyping approaches to complex dietary datasets, and the utility of genomic analysis to understand the relationships between diet and human health. 1 Programs in Metabolism and Medical and Population Genetics, The Broad Institute of MIT and Harvard, Cambridge, MA, USA. 2 Diabetes Unit and Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA. 3 Division of Endocrinology and Center for Basic and Translational Obesity Research, Boston Children’s Hospital, Boston, MA, -
Explorations in Olfactory Receptor Structure and Function by Jianghai
Explorations in Olfactory Receptor Structure and Function by Jianghai Ho Department of Neurobiology Duke University Date:_______________________ Approved: ___________________________ Hiroaki Matsunami, Supervisor ___________________________ Jorg Grandl, Chair ___________________________ Marc Caron ___________________________ Sid Simon ___________________________ [Committee Member Name] Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Neurobiology in the Graduate School of Duke University 2014 ABSTRACT Explorations in Olfactory Receptor Structure and Function by Jianghai Ho Department of Neurobiology Duke University Date:_______________________ Approved: ___________________________ Hiroaki Matsunami, Supervisor ___________________________ Jorg Grandl, Chair ___________________________ Marc Caron ___________________________ Sid Simon ___________________________ [Committee Member Name] An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Neurobiology in the Graduate School of Duke University 2014 Copyright by Jianghai Ho 2014 Abstract Olfaction is one of the most primitive of our senses, and the olfactory receptors that mediate this very important chemical sense comprise the largest family of genes in the mammalian genome. It is therefore surprising that we understand so little of how olfactory receptors work. In particular we have a poor idea of what chemicals are detected by most of the olfactory receptors in the genome, and for those receptors which we have paired with ligands, we know relatively little about how the structure of these ligands can either activate or inhibit the activation of these receptors. Furthermore the large repertoire of olfactory receptors, which belong to the G protein coupled receptor (GPCR) superfamily, can serve as a model to contribute to our broader understanding of GPCR-ligand binding, especially since GPCRs are important pharmaceutical targets. -
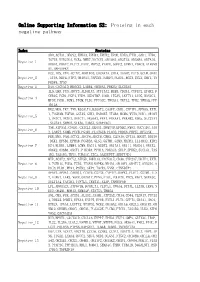
Online Supporting Information S2: Proteins in Each Negative Pathway
Online Supporting Information S2: Proteins in each negative pathway Index Proteins ADO,ACTA1,DEGS2,EPHA3,EPHB4,EPHX2,EPOR,EREG,FTH1,GAD1,HTR6, IGF1R,KIR2DL4,NCR3,NME7,NOTCH1,OR10S1,OR2T33,OR56B4,OR7A10, Negative_1 OR8G1,PDGFC,PLCZ1,PROC,PRPS2,PTAFR,SGPP2,STMN1,VDAC3,ATP6V0 A1,MAPKAPK2 DCC,IDS,VTN,ACTN2,AKR1B10,CACNA1A,CHIA,DAAM2,FUT5,GCLM,GNAZ Negative_2 ,ITPA,NEU4,NTF3,OR10A3,PAPSS1,PARD3,PLOD1,RGS3,SCLY,SHC1,TN FRSF4,TP53 Negative_3 DAO,CACNA1D,HMGCS2,LAMB4,OR56A3,PRKCQ,SLC25A5 IL5,LHB,PGD,ADCY3,ALDH1A3,ATP13A2,BUB3,CD244,CYFIP2,EPHX2,F CER1G,FGD1,FGF4,FZD9,HSD17B7,IL6R,ITGAV,LEFTY1,LIPG,MAN1C1, Negative_4 MPDZ,PGM1,PGM3,PIGM,PLD1,PPP3CC,TBXAS1,TKTL2,TPH2,YWHAQ,PPP 1R12A HK2,MOS,TKT,TNN,B3GALT4,B3GAT3,CASP7,CDH1,CYFIP1,EFNA5,EXTL 1,FCGR3B,FGF20,GSTA5,GUK1,HSD3B7,ITGB4,MCM6,MYH3,NOD1,OR10H Negative_5 1,OR1C1,OR1E1,OR4C11,OR56A3,PPA1,PRKAA1,PRKAB2,RDH5,SLC27A1 ,SLC2A4,SMPD2,STK36,THBS1,SERPINC1 TNR,ATP5A1,CNGB1,CX3CL1,DEGS1,DNMT3B,EFNB2,FMO2,GUCY1B3,JAG Negative_6 2,LARS2,NUMB,PCCB,PGAM1,PLA2G1B,PLOD2,PRDX6,PRPS1,RFXANK FER,MVD,PAH,ACTC1,ADCY4,ADCY8,CBR3,CLDN16,CPT1A,DDOST,DDX56 ,DKK1,EFNB1,EPHA8,FCGR3A,GLS2,GSTM1,GZMB,HADHA,IL13RA2,KIR2 Negative_7 DS4,KLRK1,LAMB4,LGMN,MAGI1,NUDT2,OR13A1,OR1I1,OR4D11,OR4X2, OR6K2,OR8B4,OXCT1,PIK3R4,PPM1A,PRKAG3,SELP,SPHK2,SUCLG1,TAS 1R2,TAS1R3,THY1,TUBA1C,ZIC2,AASDHPPT,SERPIND1 MTR,ACAT2,ADCY2,ATP5D,BMPR1A,CACNA1E,CD38,CYP2A7,DDIT4,EXTL Negative_8 1,FCER1G,FGD3,FZD5,ITGAM,MAPK8,NR4A1,OR10V1,OR4F17,OR52D1,O R8J3,PLD1,PPA1,PSEN2,SKP1,TACR3,VNN1,CTNNBIP1 APAF1,APOA1,CARD11,CCDC6,CSF3R,CYP4F2,DAPK1,FLOT1,GSTM1,IL2 -
Get High-Res Image
25 Syn. Frame shift 20 Missense In frame inA94 Syn. 15 Splice site Other non syn. Non syn. 10 Nonsense 5 # mutations/#< 0 239 50% 1HL 143 31% $- 5% DNMT1 4% 'IAA0408 4% CCDC136 4% SMG7 3% CCDC91 3% ARA$3 3% D$$3 3% TAS2R3 3% ACSL3 3% !FN-3 3% NAPSA 3% (SB$L3 3% SPD2E3 2% C1S 2% $KD2 2% SLC26A4 2% TSKS 2% $"2" 2% (R4A47 2% $&!$ 2% SDHAF2 2% SLC4A5 2% A&$C2 2% CHS21 2% $DE9A 2% &RAS2 2% T&" 2% NET(2 2% $CG 1 2% &-$JL 2% SHANK1 2% ZMAT2 1% AP 1% -*(24 1% NSFL1C 1% N+D1 1% $&-1 1% $*#$2 1% SLC35 1 1% D"&S* 1% !RN2 1% (R9,1 1% $AX2 1% &ASAL1 1% &NF168 1% SNRNP70 1% TATDN2 1% -#$8A 1% C$-2 1% G) 1 1% #$( 53 11% SETD2 4% $TEN 6% 'DM5C 5% -AP1 3% TP53 37 7% #TO& 2% S$&24 2% S(&D 2% (R4D1 2% SLC6A7 2% C%-3 3% LET#1 2% NAT8 2% AM122C 2% !XTL2 2% !GR1 2% #AR'4 2% #&$L10 2% $CG 2 79 11% #UC4 2% DCA 11 1% CDC42EP3 2% SLC30A9 1% -# 2% CEC&1 2% CCDC97 1% C$(* 2% CDH&1 2% LET#D1 3% CAMTA2 1% TSPAN19 2% D$!$2 2% ZNF280A 1% NPL 1% !#$2 2% "PS3 2% $LOD3 1% ZNF512 1% T%#P3 1% SLC43A3 2% ZNF625 1% TCE-1 1% LDLRA$1 1% ANK&D23 1% SLC43A1 2% CD4 2% ZDH"C5 2% ANG$TL6 1% %$6K2 2% &-#23 1% IGSF5 2% &PS6KA1 1% 2% 1A 1% -RA$ 2% NACA 2% A#"&2 1% C&2-B1 1% 1PS33A 1% ATP2A3 2% ABCC1 1% G#!-1 1% )-*N7 1% (R4 15 2% DCDC1 1% !MG1 1% T#!#229A 0% (R2A25 1% $OL&2F 2% N! " 1% (R4D11 2% SCN4A 2% TBC1D8- 1% 'IAA1919 1% !LO1L5 1% TES'1 1% S!#A4F 2% T"-S1 1% $&'AA2 1% SLC18A2 1% $TG!R3 3% DNMT3A 1% #TF&1 1% TTF2 1% #'* 1% !DIL3 1% ZAD"2 1% TAGLN3 0% C4orf47 1% ASRGL1 2% ATN1 2% ZBTB39 1% (R4'5 2% T#!#145 4% &AN-P2 1% NCK%$SD 2% AGA$3 2% N !2L2 1% A2ML1 2% $%'3CA 2% $RD#4 1% -

Supplementary Methods
doi: 10.1038/nature06162 SUPPLEMENTARY INFORMATION Supplementary Methods Cloning of human odorant receptors 423 human odorant receptors were cloned with sequence information from The Olfactory Receptor Database (http://senselab.med.yale.edu/senselab/ORDB/default.asp). Of these, 335 were predicted to encode functional receptors, 45 were predicted to encode pseudogenes, 29 were putative variant pairs of the same genes, and 14 were duplicates. We adopted the nomenclature proposed by Doron Lancet 1. OR7D4 and the six intact odorant receptor genes in the OR7D4 gene cluster (OR1M1, OR7G2, OR7G1, OR7G3, OR7D2, and OR7E24) were used for functional analyses. SNPs in these odorant receptors were identified from the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP) or through genotyping. OR7D4 single nucleotide variants were generated by cloning the reference sequence from a subject or by inducing polymorphic SNPs by site-directed mutagenesis using overlap extension PCR. Single nucleotide and frameshift variants for the six intact odorant receptors in the same gene cluster as OR7D4 were generated by cloning the respective genes from the genomic DNA of each subject. The chimpanzee OR7D4 orthologue was amplified from chimpanzee genomic DNA (Coriell Cell Repositories). Odorant receptors that contain the first 20 amino acids of human rhodopsin tag 2 in pCI (Promega) were expressed in the Hana3A cell line along with a short form of mRTP1 called RTP1S, (M37 to the C-terminal end), which enhances functional expression of the odorant receptors 3. For experiments with untagged odorant receptors, OR7D4 RT and S84N variants without the Rho tag were cloned into pCI. -

1 Reference Sequence Number Gene Symbol Alleles Chromosomal
1 Supplemental Table 1. 10,177 non-synonymous single nucleotide polymorphisms on ParAllele panel; the San Francisco Bay Area Adult Glioma Study Minor Allele Minor Allele Reference Sequence Chromosomal Frequency, Frequency, Number Gene Symbol Alleles Location Minor Allele Case Control P-value* rs8289 FUT6, M6PRBP1 A/G 19p A 0.357798165 0.188073394 0.000141 rs10495960 LHCGR, GTF2A1L A/G 02p A 0.102678571 0.245454545 0.000145 rs1195889 GPR133 A/G 12q A 0.339285714 0.495495495 0.000547 rs11076585 C/T 16q T 0.147321429 0.290178571 0.00055 rs10415562 OR7C1 C/T 19p C 0.294642857 0.183035714 0.000805 rs7955866 FGF23 A/G 12p A 0.163636364 0.049549549 0.001078 rs3765083 MFSD1 A/G 03q A 0.486607143 0.339285714 0.001137 rs12455859 G/T 18p G 0.473214286 0.366071429 0.001248 rs3732548 hCG_1813818 A/C 03p C 0.21875 0.357142857 0.001609 rs1047840 EXO1 A/G 01q A 0.321428571 0.468181818 0.002261 rs10817025 SVEP1 C/T 09q C 0.403061224 0.252747253 0.002461 rs1265054 C6orf15 A/G 06p A 0.382882883 0.468468468 0.002688 rs319522 GPC6 A/G 13q G 0.40625 0.455357143 0.003242 rs7522157 CLCC1, C1orf62 C/T 01p T 0.254464286 0.382882883 0.003275 rs2017467 A/G 11q A 0.068181818 0.155963303 0.003282 rs4272850 TMEM132C A/G 12q A 0.294642857 0.178571429 0.003348 rs10490923 ARMS2 A/G 10q A 0.09375 0.196428571 0.003436 rs1129770 CMYA5 A/G 05q A 0.142857143 0.258928571 0.003448 rs2298771 SCN1A A/G 02q G 0.361607143 0.232142857 0.003505 rs6902416 TRDN C/G 06q G 0.1875 0.09009009 0.004266 rs2502601 SYNJ2 C/T 06q T 0.40625 0.459821429 0.0043 rs4691212 G/T 04q G 0.267857143 0.392857143