Explorations in Olfactory Receptor Structure and Function by Jianghai
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

“A Very Stinking Herbe” Is Cilantro Love/Hate a Genetic Trait? So Fresh Or So Clean? Methods Results and Discussion Referenc
A Genetic Variant Near Olfactory Receptor Genes Associates With Cilantro Preference S. Wu, J.Y. Tung, A.B. Chowdry, A.K. Kiefer, J.L. Mountain, N. Eriksson 23andMe, Inc., Mountain View, CA “A very stinking herbe”! Many people love the taste of fresh cilantro (Coriandrum sativum, also called coriander), while Oh, coriander.! others despise it with every fiber of their being. The debate has raged for centuries. Pliny extolled If "pure evil# had a taste?! its “refreshing” properties, a medieval herbalist claimed that the leaves had a “venomous quality,” It would taste like you.! by SilverSpoon at and, more recently, beloved culinary icon Julia Child opined that cilantro had “kind of a dead taste” IHateCilantro.com! and that she would “pick it out if [she] saw it and throw it on the floor.”! So fresh or so clean?! Is cilantro love/hate a genetic trait?! Cilantro"s dual nature may lie in its key aroma components, which consist of It is not known why cilantro is so differentially perceived. Cilantro various aldehydes. The unsaturated aldehydes (mostly decanal and preference varies with ancestry and may be influenced by dodecanal) are described as fruity, green, and pungent; the (E)-2-alkenals exposure. Genetic factors are also thought to play a role but to as soapy, fatty, pungent, and “like cilantro.” Of the many descriptors that date no studies have found genetic variants influencing cilantro “haters” use, soapy is the most common.! taste preference. ! Methods! Results and discussion! We conducted the first-ever genome-wide association study of cilantro preference. Participants were drawn from the more than 180,000 genotyped customers of 23andMe, Inc., a consumer genetics company.! Phenotype data collection! Participants reported their age and answered Figure 1. -

Genetic Variation Across the Human Olfactory Receptor Repertoire Alters Odor Perception
bioRxiv preprint doi: https://doi.org/10.1101/212431; this version posted November 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Genetic variation across the human olfactory receptor repertoire alters odor perception Casey Trimmer1,*, Andreas Keller2, Nicolle R. Murphy1, Lindsey L. Snyder1, Jason R. Willer3, Maira Nagai4,5, Nicholas Katsanis3, Leslie B. Vosshall2,6,7, Hiroaki Matsunami4,8, and Joel D. Mainland1,9 1Monell Chemical Senses Center, Philadelphia, Pennsylvania, USA 2Laboratory of Neurogenetics and Behavior, The Rockefeller University, New York, New York, USA 3Center for Human Disease Modeling, Duke University Medical Center, Durham, North Carolina, USA 4Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, North Carolina, USA 5Department of Biochemistry, University of Sao Paulo, Sao Paulo, Brazil 6Howard Hughes Medical Institute, New York, New York, USA 7Kavli Neural Systems Institute, New York, New York, USA 8Department of Neurobiology and Duke Institute for Brain Sciences, Duke University Medical Center, Durham, North Carolina, USA 9Department of Neuroscience, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA *[email protected] ABSTRACT The human olfactory receptor repertoire is characterized by an abundance of genetic variation that affects receptor response, but the perceptual effects of this variation are unclear. To address this issue, we sequenced the OR repertoire in 332 individuals and examined the relationship between genetic variation and 276 olfactory phenotypes, including the perceived intensity and pleasantness of 68 odorants at two concentrations, detection thresholds of three odorants, and general olfactory acuity. -

Database Tool the Systematic Annotation of the Three Main GPCR
Database, Vol. 2010, Article ID baq018, doi:10.1093/database/baq018 ............................................................................................................................................................................................................................................................................................. Database tool The systematic annotation of the three main Downloaded from https://academic.oup.com/database/article-abstract/doi/10.1093/database/baq018/406672 by guest on 15 January 2019 GPCR families in Reactome Bijay Jassal1, Steven Jupe1, Michael Caudy2, Ewan Birney1, Lincoln Stein2, Henning Hermjakob1 and Peter D’Eustachio3,* 1European Bioinformatics Institute, Hinxton, Cambridge, CB10 1SD, UK, 2Ontario Institute for Cancer Research, Toronto, ON M5G 0A3, Canada and 3New York University School of Medicine, New York, NY 10016, USA *Corresponding author: Tel: +212 263 5779; Fax: +212 263 8166; Email: [email protected] Submitted 14 April 2010; Revised 14 June 2010; Accepted 13 July 2010 ............................................................................................................................................................................................................................................................................................. Reactome is an open-source, freely available database of human biological pathways and processes. A major goal of our work is to provide an integrated view of cellular signalling processes that spans from ligand–receptor -

Olfactory Receptors Involved in the Perception
(19) TZZ¥ZZ__T (11) EP 3 004 157 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07K 14/705 (2006.01) C07K 14/72 (2006.01) 01.11.2017 Bulletin 2017/44 G01N 33/50 (2006.01) G01N 33/566 (2006.01) G01N 33/68 (2006.01) (21) Application number: 13730138.8 (86) International application number: (22) Date of filing: 31.05.2013 PCT/EP2013/061243 (87) International publication number: WO 2014/191047 (04.12.2014 Gazette 2014/49) (54) OLFACTORY RECEPTORS INVOLVED IN THE PERCEPTION OF SWEAT CARBOXYLIC ACIDS AND THE USE THEREOF AN DER WAHRNEHMUNG VON SCHWEISSCARBONSÄUREN BETEILIGTE GERUCHSREZEPTOREN UND VERWENDUNG DAVON RÉCEPTEURS OLFACTIFS IMPLIQUÉS DANS LA PERCEPTION D’ACIDES CARBOXYLIQUES DE SUEUR ET LEUR UTILISATION (84) Designated Contracting States: • VEITHEN, Alex AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 1476 Genappe (BE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (74) Representative: Vanhalst, Koen et al De Clercq & Partners cvba (43) Date of publication of application: E. Gevaertdreef 10a 13.04.2016 Bulletin 2016/15 9830 Sint-Martens-Latem (BE) (73) Proprietor: ChemCom S.A. (56) References cited: 1070 Brussels (BE) WO-A1-2012/029922 WO-A2-01/27158 WO-A2-2006/094704 US-A1- 2003 207 337 (72) Inventors: • CHATELAIN, Pierre 1150 Brussels (BE) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

OR10G4 (NM 001004462) Human Tagged ORF Clone Lentiviral Particle Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC222878L3V OR10G4 (NM_001004462) Human Tagged ORF Clone Lentiviral Particle Product data: Product Type: Lentiviral Particles Product Name: OR10G4 (NM_001004462) Human Tagged ORF Clone Lentiviral Particle Symbol: OR10G4 Synonyms: OR11-278 Vector: pLenti-C-Myc-DDK-P2A-Puro (PS100092) ACCN: NM_001004462 ORF Size: 933 bp ORF Nucleotide The ORF insert of this clone is exactly the same as(RC222878). Sequence: OTI Disclaimer: The molecular sequence of this clone aligns with the gene accession number as a point of reference only. However, individual transcript sequences of the same gene can differ through naturally occurring variations (e.g. polymorphisms), each with its own valid existence. This clone is substantially in agreement with the reference, but a complete review of all prevailing variants is recommended prior to use. More info OTI Annotation: This clone was engineered to express the complete ORF with an expression tag. Expression varies depending on the nature of the gene. RefSeq: NM_001004462.1, NP_001004462.1 RefSeq Size: 936 bp RefSeq ORF: 936 bp Locus ID: 390264 UniProt ID: Q8NGN3, A0A126GWS5 Protein Families: Transmembrane Protein Pathways: Olfactory transduction MW: 34.4 kDa This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 OR10G4 (NM_001004462) Human Tagged ORF Clone Lentiviral Particle – RC222878L3V Gene Summary: Olfactory receptors interact with odorant molecules in the nose, to initiate a neuronal response that triggers the perception of a smell. -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Greg's Awesome Thesis
Analysis of alignment error and sitewise constraint in mammalian comparative genomics Gregory Jordan European Bioinformatics Institute University of Cambridge A dissertation submitted for the degree of Doctor of Philosophy November 30, 2011 To my parents, who kept us thinking and playing This dissertation is the result of my own work and includes nothing which is the out- come of work done in collaboration except where specifically indicated in the text and acknowledgements. This dissertation is not substantially the same as any I have submitted for a degree, diploma or other qualification at any other university, and no part has already been, or is currently being submitted for any degree, diploma or other qualification. This dissertation does not exceed the specified length limit of 60,000 words as defined by the Biology Degree Committee. November 30, 2011 Gregory Jordan ii Analysis of alignment error and sitewise constraint in mammalian comparative genomics Summary Gregory Jordan November 30, 2011 Darwin College Insight into the evolution of protein-coding genes can be gained from the use of phylogenetic codon models. Recently sequenced mammalian genomes and powerful analysis methods developed over the past decade provide the potential to globally measure the impact of natural selection on pro- tein sequences at a fine scale. The detection of positive selection in particular is of great interest, with relevance to the study of host-parasite conflicts, immune system evolution and adaptive dif- ferences between species. This thesis examines the performance of methods for detecting positive selection first with a series of simulation experiments, and then with two empirical studies in mammals and primates. -

Whole Exome Sequencing in Families at High Risk for Hodgkin Lymphoma: Identification of a Predisposing Mutation in the KDR Gene
Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene Melissa Rotunno, 1 Mary L. McMaster, 1 Joseph Boland, 2 Sara Bass, 2 Xijun Zhang, 2 Laurie Burdett, 2 Belynda Hicks, 2 Sarangan Ravichandran, 3 Brian T. Luke, 3 Meredith Yeager, 2 Laura Fontaine, 4 Paula L. Hyland, 1 Alisa M. Goldstein, 1 NCI DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, Stephen J. Chanock, 5 Neil E. Caporaso, 1 Margaret A. Tucker, 6 and Lynn R. Goldin 1 1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 2Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 3Ad - vanced Biomedical Computing Center, Leidos Biomedical Research Inc.; Frederick National Laboratory for Cancer Research, Frederick, MD; 4Westat, Inc., Rockville MD; 5Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; and 6Human Genetics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA ©2016 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.135475 Received: August 19, 2015. Accepted: January 7, 2016. Pre-published: June 13, 2016. Correspondence: [email protected] Supplemental Author Information: NCI DCEG Cancer Sequencing Working Group: Mark H. Greene, Allan Hildesheim, Nan Hu, Maria Theresa Landi, Jennifer Loud, Phuong Mai, Lisa Mirabello, Lindsay Morton, Dilys Parry, Anand Pathak, Douglas R. Stewart, Philip R. Taylor, Geoffrey S. Tobias, Xiaohong R. Yang, Guoqin Yu NCI DCEG Cancer Genomics Research Laboratory: Salma Chowdhury, Michael Cullen, Casey Dagnall, Herbert Higson, Amy A. -

Time-Series Plasma Cell-Free DNA Analysis Reveals Disease Severity of COVID-19 Patients
medRxiv preprint doi: https://doi.org/10.1101/2020.06.08.20124305; this version posted June 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Time-series plasma cell-free DNA analysis reveals disease severity of COVID- 19 patients Authors: Xinping Chen1†, Yu Lin2†, Tao Wu1†, Jinjin Xu2†, Zhichao Ma1†, Kun Sun2,5†, Hui Li1†, Yuxue Luo2,3†, Chen Zhang1, Fang Chen2, Jiao Wang1, Tingyu Kuo2,4, Xiaojuan Li1, Chunyu Geng2, Feng Lin1, Chaojie Huang2, Junjie Hu1, Jianhua Yin2, Ming Liu1, Ye Tao2, Jiye Zhang1, Rijing Ou2, Furong Xiao1, Huanming Yang2,6, Jian Wang2,6, Xun Xu2,7, Shengmiao Fu1*, Xin Jin2,3*, Hongyan Jiang1*, Ruoyan Chen2* Affiliations: 1Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Hainan Provincial Key Laboratory of Cell and Molecular Genetic Translational Medicine, Haikou 570311, Hainan, China. 2BGI-Shenzhen, Shenzhen, 518083, Guangdong, China 3School of Medicine, South China University of Technology, Guangzhou 510006, Guangdong, China 4BGI Education Center, University of Chinese Academy of Sciences, Shenzhen 518083, Guangdong, China 5Shenzhen Bay Laboratory, Shenzhen 518132, Guangdong, China 6James D. Watson Institute of Genome Sciences, Hangzhou 310058, China 7Guangdong Provincial Key Laboratory of Genome Read and Write, BGI-Shenzhen, Shenzhen, 518120, China *Correspondence to: [email protected]; [email protected]; [email protected]; [email protected]. †These authors contributed equally to this work. Abstract: Clinical symptoms of coronavirus disease 2019 (COVID-19) range from asymptomatic to severe pneumonia and death. -

Deep Sequencing of the Human Retinae Reveals the Expression of Odorant Receptors
fncel-11-00003 January 20, 2017 Time: 14:24 # 1 CORE Metadata, citation and similar papers at core.ac.uk Provided by Frontiers - Publisher Connector ORIGINAL RESEARCH published: 24 January 2017 doi: 10.3389/fncel.2017.00003 Deep Sequencing of the Human Retinae Reveals the Expression of Odorant Receptors Nikolina Jovancevic1*, Kirsten A. Wunderlich2, Claudia Haering1, Caroline Flegel1, Désirée Maßberg1, Markus Weinrich1, Lea Weber1, Lars Tebbe2, Anselm Kampik3, Günter Gisselmann1, Uwe Wolfrum2, Hanns Hatt1† and Lian Gelis1† 1 Department of Cell Physiology, Ruhr-University Bochum, Bochum, Germany, 2 Department of Cell and Matrix Biology, Johannes Gutenberg University of Mainz, Mainz, Germany, 3 Department of Ophthalmology, Ludwig Maximilian University of Munich, Munich, Germany Several studies have demonstrated that the expression of odorant receptors (ORs) occurs in various tissues. These findings have served as a basis for functional studies that demonstrate the potential of ORs as drug targets for a clinical application. To the best of our knowledge, this report describes the first evaluation of the mRNA expression of ORs and the localization of OR proteins in the human retina that set a Edited by: stage for subsequent functional analyses. RNA-Sequencing datasets of three individual Hansen Wang, University of Toronto, Canada neural retinae were generated using Next-generation sequencing and were compared Reviewed by: to previously published but reanalyzed datasets of the peripheral and the macular Ewald Grosse-Wilde, human retina and to reference tissues. The protein localization of several ORs was Max Planck Institute for Chemical Ecology (MPG), Germany investigated by immunohistochemistry. The transcriptome analyses detected an average Takaaki Sato, of 14 OR transcripts in the neural retina, of which OR6B3 is one of the most highly National Institute of Advanced expressed ORs. -

An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors
Ecology and Evolutionary Biology 2021; 6(3): 53-77 http://www.sciencepublishinggroup.com/j/eeb doi: 10.11648/j.eeb.20210603.11 ISSN: 2575-3789 (Print); ISSN: 2575-3762 (Online) An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors Miguel Angel Fuertes*, Carlos Alonso Department of Microbiology, Centre for Molecular Biology “Severo Ochoa”, Spanish National Research Council and Autonomous University, Madrid, Spain Email address: *Corresponding author To cite this article: Miguel Angel Fuertes, Carlos Alonso. An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors. Ecology and Evolutionary Biology. Vol. 6, No. 3, 2021, pp. 53-77. doi: 10.11648/j.eeb.20210603.11 Received: April 24, 2021; Accepted: May 11, 2021; Published: July 13, 2021 Abstract: Capturing conserved patterns in genes and proteins is important for inferring phenotype prediction and evolutionary analysis. The study is focused on the conserved patterns of the G protein-coupled receptors, an important superfamily of receptors. Olfactory receptors represent more than 2% of our genome and constitute the largest family of G protein-coupled receptors, a key class of drug targets. As no crystallographic structures are available, mechanistic studies rely on the use of molecular dynamic modelling combined with site-directed mutagenesis data. In this paper, we hypothesized that human-mouse orthologs coding for G protein-coupled receptors maintain, at speciation events, shared compositional structures independent, to some extent, of their percent identity as reveals a method based in the categorization of nucleotide triplets by their gross composition. The data support the consistency of the hypothesis, showing in ortholog G protein-coupled receptors the presence of emergent shared compositional structures preserved at speciation events. -
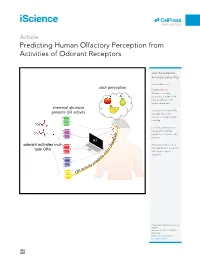
Predicting Human Olfactory Perception from Activities of Odorant Receptors
iScience ll OPEN ACCESS Article Predicting Human Olfactory Perception from Activities of Odorant Receptors Joel Kowalewski, Anandasankar Ray [email protected] odor perception HIGHLIGHTS Machine learning predicted activity of 34 human ORs for ~0.5 million chemicals chemical structure Activities of human ORs predicts OR activity could predict odor character using machine learning Few OR activities were needed to optimize r predictions of each odor e t c percept a AI r a odorant activates mul- h Behavior predictions in c Drosophila also need few r tiple ORs o olfactory receptor d o activities ts ic ed pr ity tiv ac OR Kowalewski & Ray, iScience 23, 101361 August 21, 2020 ª 2020 The Author(s). https://doi.org/10.1016/ j.isci.2020.101361 iScience ll OPEN ACCESS Article Predicting Human Olfactory Perception from Activities of Odorant Receptors Joel Kowalewski1 and Anandasankar Ray1,2,3,* SUMMARY Odor perception in humans is initiated by activation of odorant receptors (ORs) in the nose. However, the ORs linked to specific olfactory percepts are unknown, unlike in vision or taste where receptors are linked to perception of different colors and tastes. The large family of ORs (~400) and multiple receptors activated by an odorant present serious challenges. Here, we first use machine learning to screen ~0.5 million compounds for new ligands and identify enriched structural motifs for ligands of 34 human ORs. We next demonstrate that the activity of ORs successfully predicts many of the 146 different perceptual qualities of chem- icals. Although chemical features have been used to model odor percepts, we show that biologically relevant OR activity is often superior.