A Unique Coral Biomineralization Pattern Has Resisted 40 Million Years of Major Ocean Chemistry Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Coral Reefs, Biodiversity, Distribution Patterns, Persian Gulf
Journal of Survey in Fisheries Sciences 1(2)21-26 2015 Biodiversity and distribution patterns of coral reef ecosystems in ROPME Sea Area (Inner part: Persian Gulf -Iranian waters) 1* 1 2 3 Owfi F, ; Rabbaniha M. ; Al-Obeid Mehana S. ; Mahichi F. Received: July 2014 Accepted: November 2014 Abstract The Persian Gulf is northern part of the ROPME Sea Area (RSA), and is semi-enclosed shallow sea which located in subtropical climate. Measuring is 1000km in length, varying in width 60-340km, and average depth is about 35m and maximum depth is 105m. This research was carried out during 2005-2010 for reviewing the corals status and determination of coral reef habitats distribution in the Persian Gulf. Coral reef sites and patches were determined by field surveys and direct observations. Also, the violet-blue bands of Land Sat satellite images were used for seaward extent of coral reefs. Sampling sites covered by SCUBA, Manta Tow technique and Transect survey base on Reef Check and GCRMN standard method. Coral reefs habitats in Iranian coastal areas are found in two types and structures which observed around seventeen islands fringing coral reefs and two limited patches of coral reefs around Nayband Bay. Widest coral reef cover observed around Khark, Kharko and Larak Islands. Due to extremes of water temperature and salinity that are close to the physiological tolerance limits ,coral reefs diversity and coral species composition in this area is similar and close to Indo-Pacific eco-region coral communities. In comparison, species richness of hard corals in the Gulf of Oman; with about 70 species is more than the entire Persian Gulf. -

Scleractinian Reef Corals: Identification Notes
SCLERACTINIAN REEF CORALS: IDENTIFICATION NOTES By JACKIE WOLSTENHOLME James Cook University AUGUST 2004 DOI: 10.13140/RG.2.2.24656.51205 http://dx.doi.org/10.13140/RG.2.2.24656.51205 Scleractinian Reef Corals: Identification Notes by Jackie Wolstenholme is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................................................................ i INTRODUCTION .................................................................................................................................................. 1 ABBREVIATIONS AND DEFINITIONS ............................................................................................................. 2 FAMILY ACROPORIDAE.................................................................................................................................... 3 Montipora ........................................................................................................................................................... 3 Massive/thick plates/encrusting & tuberculae/papillae ................................................................................... 3 Montipora monasteriata .............................................................................................................................. 3 Massive/thick plates/encrusting & papillae ................................................................................................... -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -
![Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]](https://docslib.b-cdn.net/cover/7255/ornamental-fish-and-marine-invertebrates-draft-for-consultation-document-date-1037255.webp)
Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]
Ornamental Fish and Marine Invertebrates ORNAMARI.ALL [Document Date] Health Standard Import Import Issued under the Biosecurity Act 1993 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] TITLE Import Health Standard: Ornamental Fish and Marine Invertebrates COMMENCEMENT This Import Health Standard comes into force on [Effective Date] REVOCATION This Import Health Standard revokes and replaces: Import Health Standard for Ornamental Fish and Marine Invertebrates from all countries, 20 April 2011. ISSUING AUTHORITY This Import Health Standard is issued on Dated at Wellington this ... day of ......... Howard Pharo Manager, Import and Export Animals Ministry for Primary Industries (acting under delegated authority of the Director-General) Contact for further information Ministry for Primary Industries (MPI) Regulation & Assurance Branch Animal Imports PO Box 2526 Wellington 6140 Email: [email protected] Ministry for Primary Industries Page 1 of 75 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] Contents Page Introduction 4 Part 1: Requirements 6 1.1 Application 6 1.2 Outcome 6 1.3 Incorporation by reference 7 1.4 Definitions 7 1.5 Harmonised system (HS) codes 7 1.6 Exporting country systems and certification 8 1.7 Diagnostic testing and treatment 8 1.8 Packaging 9 1.9 Import permit 9 1.10 The documentation that must accompany goods 9 1.11 Inspection and verification 10 1.12 Transitional facility 11 1.13 Pre-export isolation -

AC27 Doc. 12.5
Original language: English AC27 Doc. 12.5 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-seventh meeting of the Animals Committee Veracruz (Mexico), 28 April – 3 May 2014 Interpretation and implementation of the Convention Review of Significant Trade in specimens of Appendix-II species [Resolution Conf. 12.8 (Rev. CoP13)] SELECTION OF SPECIES FOR TRADE REVIEWS FOLLOWING COP16 1. This document has been prepared by the Secretariat. 2. In Resolution Conf. 12.8 (Rev. CoP13) on Review of Significant Trade in specimens of Appendix-II species, the Conference of the Parties: DIRECTS the Animals and Plants Committees, in cooperation with the Secretariat and experts, and in consultation with range States, to review the biological, trade and other relevant information on Appendix-II species subject to significant levels of trade, to identify problems and solutions concerning the implementation of Article IV, paragraphs 2 (a), 3 and 6 (a)... 3. In accordance with paragraph a) of that Resolution under the section Regarding conduct of the Review of Significant Trade, the Secretariat requested UNEP-WCMC to produce a summary from the CITES Trade Database of annual report statistics showing the recorded net level of exports for Appendix-II species over the five most recent years. Its report is attached as Annex 1 (English only) to the present document. The raw data used to prepare this summary are available in document AC27 Inf. 2. 4. Paragraph b) of the same section directs the Animals Committee, on the basis of recorded trade levels and information available to it, the Secretariat, Parties or other relevant experts, to select species of priority concern for review (whether or not such species have been the subject of a previous review). -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -

AC26 Doc. 20 Annex 6 English Only / Únicamente En Inglés / Seulement En Anglais
AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais Annex 6 CORAL SPECIES, SYNONYMS, AND NOMENCLATURE REFERENCES CURRENTLY RECOGNIZED IN THE UNEP-WCMC DATABASE Data kindly provided by UNEP-WCMC AC26 Doc. 20, Annex 6 – 1 AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families FamName Accepted Name SpcAuthor Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Acropora mangarevensis; Madrepora ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) crassa; Madrepora abrotanoides ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Acropora diffusa; Madrepora acuminata; ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Madrepora diffusa; Madrepora nigra ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora anthocercis; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) coronata ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Acropora cribripora; Acropora hebes; Acropora manni; Acropora yaeyamaensis; ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Madrepora aspera; Madrepora cribripora; Madrepora hebes; Madrepora manni ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, -
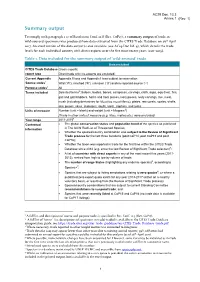
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Examining the Effect of Heat Stress on Montastraea Cavernosa
water Article Examining the Effect of Heat Stress on Montastraea cavernosa (Linnaeus 1767) from a Mesophotic Coral Ecosystem (MCE) John E. Skutnik 1, Sango Otieno 2 , Sok Kean Khoo 3 and Kevin B. Strychar 1,* 1 Annis Water Resources Institute, Grand Valley State University, Muskegon, MI 49441, USA; [email protected] 2 Department of Statistics, Grand Valley State University, Allendale, MI 49401, USA; [email protected] 3 Department Cell and Molecular Biology, Grand Valley State University, Allendale, MI 49401, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-616-331-8796 Received: 13 March 2020; Accepted: 29 April 2020; Published: 5 May 2020 Abstract: Coral reefs are under increasing pressure from global warming. Little knowledge, however, exists regarding heat induced stress on deeper mesophotic coral ecosystems (MCEs). Here, we examined the effect of acute (72 h) and chronic (480 h) heat stress on the host coral Montastraea cavernosa (Linnaeus 1767) collected from an upper MCE (~30 m) in Florida, USA. We examined six immune/stress-related genes: ribosomal protein L9 (RpL9), ribosomal protein S7 (RpS7), B-cell lymphoma 2 apoptosis regulator (BCL-2), heat shock protein 90 (HSP90), catalase, and cathepsin L1, as a proxy for coral response to heat stress. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to evaluate the gene expression. Overall, both acute and chronic heat stress treatments elicited a response in gene expression relative to control samples. Acute heat exposure resulted in up-regulation of catalase, BCL-2, and HSP90 at all time points from hour 24 to 48, suggesting the activation of an oxidative protective enzyme, molecular chaperone, and anti-apoptotic protein.