Marine Ecology Progress Series 439:117
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

A Unique Coral Biomineralization Pattern Has Resisted 40 Million Years of Major Ocean Chemistry Change
www.nature.com/scientificreports OPEN A unique coral biomineralization pattern has resisted 40 million years of major ocean chemistry Received: 14 December 2015 Accepted: 17 May 2016 change Published: 15 June 2016 Jarosław Stolarski1, Francesca R. Bosellini2, Carden C. Wallace3, Anne M. Gothmann4, Maciej Mazur5, Isabelle Domart-Coulon6, Eldad Gutner-Hoch7, Rolf D. Neuser8, Oren Levy7, Aldo Shemesh9 & Anders Meibom10,11 Today coral reefs are threatened by changes to seawater conditions associated with rapid anthropogenic global climate change. Yet, since the Cenozoic, these organisms have experienced major fluctuations in atmospheric CO2 levels (from greenhouse conditions of high pCO2 in the Eocene to low pCO2 ice-house conditions in the Oligocene-Miocene) and a dramatically changing ocean Mg/Ca ratio. Here we show that the most diverse, widespread, and abundant reef-building coral genus Acropora (20 morphological groups and 150 living species) has not only survived these environmental changes, but has maintained its distinct skeletal biomineralization pattern for at least 40 My: Well-preserved fossil Acropora skeletons from the Eocene, Oligocene, and Miocene show ultra-structures indistinguishable from those of extant representatives of the genus and their aragonitic skeleton Mg/Ca ratios trace the inferred ocean Mg/Ca ratio precisely since the Eocene. Therefore, among marine biogenic carbonate fossils, well-preserved acroporid skeletons represent material with very high potential for reconstruction of ancient ocean chemistry. Genomic sequencing has transformed our understanding of the evolution of scleractinian corals. However, the molecular clades defined for scleractinians are difficult to reconcile with traditional taxonomic classification based on overall skeletal morphology1–3. Instead, they have been shown to be broadly consistent with recently defined micro-morphological and ultrastructural skeletal criteria4–7. -
![Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]](https://docslib.b-cdn.net/cover/7255/ornamental-fish-and-marine-invertebrates-draft-for-consultation-document-date-1037255.webp)
Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]
Ornamental Fish and Marine Invertebrates ORNAMARI.ALL [Document Date] Health Standard Import Import Issued under the Biosecurity Act 1993 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] TITLE Import Health Standard: Ornamental Fish and Marine Invertebrates COMMENCEMENT This Import Health Standard comes into force on [Effective Date] REVOCATION This Import Health Standard revokes and replaces: Import Health Standard for Ornamental Fish and Marine Invertebrates from all countries, 20 April 2011. ISSUING AUTHORITY This Import Health Standard is issued on Dated at Wellington this ... day of ......... Howard Pharo Manager, Import and Export Animals Ministry for Primary Industries (acting under delegated authority of the Director-General) Contact for further information Ministry for Primary Industries (MPI) Regulation & Assurance Branch Animal Imports PO Box 2526 Wellington 6140 Email: [email protected] Ministry for Primary Industries Page 1 of 75 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] Contents Page Introduction 4 Part 1: Requirements 6 1.1 Application 6 1.2 Outcome 6 1.3 Incorporation by reference 7 1.4 Definitions 7 1.5 Harmonised system (HS) codes 7 1.6 Exporting country systems and certification 8 1.7 Diagnostic testing and treatment 8 1.8 Packaging 9 1.9 Import permit 9 1.10 The documentation that must accompany goods 9 1.11 Inspection and verification 10 1.12 Transitional facility 11 1.13 Pre-export isolation -

AC27 Doc. 12.5
Original language: English AC27 Doc. 12.5 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-seventh meeting of the Animals Committee Veracruz (Mexico), 28 April – 3 May 2014 Interpretation and implementation of the Convention Review of Significant Trade in specimens of Appendix-II species [Resolution Conf. 12.8 (Rev. CoP13)] SELECTION OF SPECIES FOR TRADE REVIEWS FOLLOWING COP16 1. This document has been prepared by the Secretariat. 2. In Resolution Conf. 12.8 (Rev. CoP13) on Review of Significant Trade in specimens of Appendix-II species, the Conference of the Parties: DIRECTS the Animals and Plants Committees, in cooperation with the Secretariat and experts, and in consultation with range States, to review the biological, trade and other relevant information on Appendix-II species subject to significant levels of trade, to identify problems and solutions concerning the implementation of Article IV, paragraphs 2 (a), 3 and 6 (a)... 3. In accordance with paragraph a) of that Resolution under the section Regarding conduct of the Review of Significant Trade, the Secretariat requested UNEP-WCMC to produce a summary from the CITES Trade Database of annual report statistics showing the recorded net level of exports for Appendix-II species over the five most recent years. Its report is attached as Annex 1 (English only) to the present document. The raw data used to prepare this summary are available in document AC27 Inf. 2. 4. Paragraph b) of the same section directs the Animals Committee, on the basis of recorded trade levels and information available to it, the Secretariat, Parties or other relevant experts, to select species of priority concern for review (whether or not such species have been the subject of a previous review). -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -

AC26 Doc. 20 Annex 6 English Only / Únicamente En Inglés / Seulement En Anglais
AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais Annex 6 CORAL SPECIES, SYNONYMS, AND NOMENCLATURE REFERENCES CURRENTLY RECOGNIZED IN THE UNEP-WCMC DATABASE Data kindly provided by UNEP-WCMC AC26 Doc. 20, Annex 6 – 1 AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families FamName Accepted Name SpcAuthor Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Acropora mangarevensis; Madrepora ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) crassa; Madrepora abrotanoides ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Acropora diffusa; Madrepora acuminata; ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Madrepora diffusa; Madrepora nigra ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora anthocercis; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) coronata ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Acropora cribripora; Acropora hebes; Acropora manni; Acropora yaeyamaensis; ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Madrepora aspera; Madrepora cribripora; Madrepora hebes; Madrepora manni ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, -
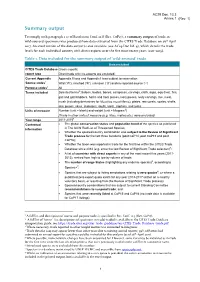
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Cop18 Doc. 99 A6
CoP18 Doc. 99 Annex 6 (English only / seulement en anglais / únicamente en inglés) Annex 6 to CoP18 Doc. 99 Nomenclature document – proposed changes in the published literature concerning nomenclature of CITES-listed animal species for which the Animals Committee, at the time of CoP18 document submission, has not yet reached a recommendation on adoption or rejection for CITES purposes. For ease of navigation, this Annex is divided into seven sections: Annex 6A: Mammals pages 2 - 8 Annex 6B: Birds (to be reviewed in conjunction with Annex 5) pages 9-36 Annex 6C: Reptiles pages 37-44 Annex 6D: Amphibians pages 45-46 Annex 6E: Cartilaginous and bony Fishes pages 47-48 Annex 6F: Invertebrates other than corals pages 49-50 Annex 6G: Corals pages 51-86 In the column ‘Appendix’, the CITES Appendix in which the species or higher taxon is listed is given; in many but not all cases, the Annex in which the species is placed in EU regulation (A, B or C) is also listed. In Annexes 6A, 6C, 6D, 6E and 6F, multiple references are sometimes cited that each document the described nomenclatural change; in those cases, individual references within the table cell are separated by ‘##’. In Annexes 6B and 6G specific symbols are used to indicate nomenclatural splits, lumps and other changes, as follows: The symbol '<' is used to indicate species lumps, i.e. taxa currently recognised as separate, but that have been grouped together as synonym or subspecies under another name in the associated reference. The symbol '>' is used to indicate species splits, i.e. -

Australia's Coral
Australia’s Coral Sea: A Biophysical Profile 2011 Dr Daniela Ceccarelli 2011 Dr Daniela Ceccarelli Coral Sea: A Biophysical Profile Australia’s Australia’s Coral Sea A Biophysical Profile Dr. Daniela Ceccarelli August 2011 Australia’s Coral Sea: A Biophysical Profile Photography credits Author: Dr. Daniela M. Ceccarelli Front and back cover: Schooling great barracuda © Jurgen Freund Dr. Daniela Ceccarelli is an independent marine ecology Page 1: South West Herald Cay, Coringa-Herald Nature Reserve © Australian Customs consultant with extensive training and experience in tropical marine ecosystems. She completed a PhD in coral reef ecology Page 2: Coral Sea © Lucy Trippett at James Cook University in 2004. Her fieldwork has taken Page 7: Masked booby © Dr. Daniela Ceccarelli her to the Great Barrier Reef and Papua New Guinea, and to remote reefs of northwest Western Australia, the Coral Sea Page 12: Humphead wrasse © Tyrone Canning and Tuvalu. In recent years she has worked as a consultant for government, non-governmental organisations, industry, Page 15: Pink anemonefish © Lucy Trippett education and research institutions on diverse projects requiring field surveys, monitoring programs, data analysis, Page 19: Hawksbill turtle © Jurgen Freund reporting, teaching, literature reviews and management recommendations. Her research and review projects have Page 21: Striped marlin © Doug Perrine SeaPics.com included studies on coral reef fish and invertebrates, Page 22: Shark and divers © Undersea Explorer seagrass beds and mangroves, and have required a good understanding of topics such as commercial shipping Page 25: Corals © Mark Spencer impacts, the effects of marine debris, the importance of apex predators, and the physical and biological attributes Page 27: Grey reef sharks © Jurgen Freund of large marine regions such as the Coral Sea. -

A Unique Coral Biomineralization Pattern Has Resisted 40 Million Years of Major Ocean Chemistry Change
www.nature.com/scientificreports OPEN A unique coral biomineralization pattern has resisted 40 million years of major ocean chemistry Received: 14 December 2015 Accepted: 17 May 2016 change Published: 15 June 2016 Jarosław Stolarski1, Francesca R. Bosellini2, Carden C. Wallace3, Anne M. Gothmann4, Maciej Mazur5, Isabelle Domart-Coulon6, Eldad Gutner-Hoch7, Rolf D. Neuser8, Oren Levy7, Aldo Shemesh9 & Anders Meibom10,11 Today coral reefs are threatened by changes to seawater conditions associated with rapid anthropogenic global climate change. Yet, since the Cenozoic, these organisms have experienced major fluctuations in atmospheric CO2 levels (from greenhouse conditions of high pCO2 in the Eocene to low pCO2 ice-house conditions in the Oligocene-Miocene) and a dramatically changing ocean Mg/Ca ratio. Here we show that the most diverse, widespread, and abundant reef-building coral genus Acropora (20 morphological groups and 150 living species) has not only survived these environmental changes, but has maintained its distinct skeletal biomineralization pattern for at least 40 My: Well-preserved fossil Acropora skeletons from the Eocene, Oligocene, and Miocene show ultra-structures indistinguishable from those of extant representatives of the genus and their aragonitic skeleton Mg/Ca ratios trace the inferred ocean Mg/Ca ratio precisely since the Eocene. Therefore, among marine biogenic carbonate fossils, well-preserved acroporid skeletons represent material with very high potential for reconstruction of ancient ocean chemistry. Genomic sequencing has transformed our understanding of the evolution of scleractinian corals. However, the molecular clades defined for scleractinians are difficult to reconcile with traditional taxonomic classification based on overall skeletal morphology1–3. Instead, they have been shown to be broadly consistent with recently defined micro-morphological and ultrastructural skeletal criteria4–7. -

NBSREA Design Cvrs V2.Pub
February 2009 TNC Pacific Island Countries Report No 1/09 Rapid Ecological Assessment Northern Bismarck Sea Papua New Guinea Technical report of survey conducted August 13 to September 7, 2006 Edited by: Richard Hamilton, Alison Green and Jeanine Almany Supported by: AP Anonymous February 2009 TNC Pacific Island Countries Report No 1/09 Rapid Ecological Assessment Northern Bismarck Sea Papua New Guinea Technical report of survey conducted August 13 to September 7, 2006 Edited by: Richard Hamilton, Alison Green and Jeanine Almany Published by: The Nature Conservancy, Indo-Pacific Resource Centre Author Contact Details: Dr. Richard Hamilton, 51 Edmondstone Street, South Brisbane, QLD 4101 Australia Email: [email protected] Suggested Citation: Hamilton, R., A. Green and J. Almany (eds.) 2009. Rapid Ecological Assessment: Northern Bismarck Sea, Papua New Guinea. Technical report of survey conducted August 13 to September 7, 2006. TNC Pacific Island Countries Report No. 1/09. © 2009, The Nature Conservancy All Rights Reserved. Reproduction for any purpose is prohibited without prior permission. Cover Photo: Manus © Gerald Allen ISBN 9980-9964-9-8 Available from: Indo-Pacific Resource Centre The Nature Conservancy 51 Edmondstone Street South Brisbane, QLD 4101 Australia Or via the worldwide web at: conserveonline.org/workspaces/pacific.island.countries.publications ii Foreword Manus and New Ireland provinces lie north of the Papua New Guinea mainland in the Bismarck Archipelago. More than half of the local communities in our provinces are coastal inhabitants, who for thousands of years have depended on marine resources for their livelihood. For coastal communities survival and prosperity is integrally linked to healthy marine ecosystems.