Evolution and Ecology of Coral Range Dynamics Under Climate Change Sun Wook Kim M.Sc., B.Sc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Corals of Rottnest Island Mscience Pty Ltd June 2012 Volume 1, Issue 1
Corals of Rottnest Island MScience Pty Ltd June 2012 Volume 1, Issue 1 MARINE RESEARCH Produced with the Assistance of the Rottnest Island Authority Cover picture courtesy of H. Shortland Jones * Several of the images in this publication are not from Rottnest and were sourced from corals.aims.gov.au courtesy of Dr JEN Veron Contents What are hard corals? ...........................................................................................4 Corals at Rottnest Island .....................................................................................5 Coral Identification .............................................................................................5 Hard corals found at Rottnest Island ...............................................................6 Faviidae ............................................................................................................7 Acroporidae and Pocilloporidae ....................................................................11 Dendrophyllidae ............................................................................................13 Mussidae ......................................................................................................15 Poritidae .......................................................................................................17 Siderastreidae ..............................................................................................19 Coral Identification using a customised key ................................................21 Terms used -

Biodiversity of the Kermadec Islands and Offshore Waters of the Kermadec Ridge: Report of a Coastal, Marine Mammal and Deep-Sea Survey (TAN1612)
Biodiversity of the Kermadec Islands and offshore waters of the Kermadec Ridge: report of a coastal, marine mammal and deep-sea survey (TAN1612) New Zealand Aquatic Environment and Biodiversity Report No. 179 Clark, M.R.; Trnski, T.; Constantine, R.; Aguirre, J.D.; Barker, J.; Betty, E.; Bowden, D.A.; Connell, A.; Duffy, C.; George, S.; Hannam, S.; Liggins, L..; Middleton, C.; Mills, S.; Pallentin, A.; Riekkola, L.; Sampey, A.; Sewell, M.; Spong, K.; Stewart, A.; Stewart, R.; Struthers, C.; van Oosterom, L. ISSN 1179-6480 (online) ISSN 1176-9440 (print) ISBN 978-1-77665-481-9 (online) ISBN 978-1-77665-482-6 (print) January 2017 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY 1 1. INTRODUCTION 3 1.1 Objectives: 3 1.2 Objective 1: Benthic offshore biodiversity 3 1.3 Objective 2: Marine mammal research 4 1.4 Objective 3: Coastal biodiversity and connectivity 5 2. METHODS 5 2.1 Survey area 5 2.2 Survey design 6 Offshore Biodiversity 6 Marine mammal sampling 8 Coastal survey 8 Station recording 8 2.3 Sampling operations 8 Multibeam mapping 8 Photographic transect survey 9 Fish and Invertebrate sampling 9 Plankton sampling 11 Catch processing 11 Environmental sampling 12 Marine mammal sampling 12 Dive sampling operations 12 Outreach 13 3. -

Scleractinian Reef Corals: Identification Notes
SCLERACTINIAN REEF CORALS: IDENTIFICATION NOTES By JACKIE WOLSTENHOLME James Cook University AUGUST 2004 DOI: 10.13140/RG.2.2.24656.51205 http://dx.doi.org/10.13140/RG.2.2.24656.51205 Scleractinian Reef Corals: Identification Notes by Jackie Wolstenholme is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................................................................ i INTRODUCTION .................................................................................................................................................. 1 ABBREVIATIONS AND DEFINITIONS ............................................................................................................. 2 FAMILY ACROPORIDAE.................................................................................................................................... 3 Montipora ........................................................................................................................................................... 3 Massive/thick plates/encrusting & tuberculae/papillae ................................................................................... 3 Montipora monasteriata .............................................................................................................................. 3 Massive/thick plates/encrusting & papillae ................................................................................................... -

A Unique Coral Biomineralization Pattern Has Resisted 40 Million Years of Major Ocean Chemistry Change
www.nature.com/scientificreports OPEN A unique coral biomineralization pattern has resisted 40 million years of major ocean chemistry Received: 14 December 2015 Accepted: 17 May 2016 change Published: 15 June 2016 Jarosław Stolarski1, Francesca R. Bosellini2, Carden C. Wallace3, Anne M. Gothmann4, Maciej Mazur5, Isabelle Domart-Coulon6, Eldad Gutner-Hoch7, Rolf D. Neuser8, Oren Levy7, Aldo Shemesh9 & Anders Meibom10,11 Today coral reefs are threatened by changes to seawater conditions associated with rapid anthropogenic global climate change. Yet, since the Cenozoic, these organisms have experienced major fluctuations in atmospheric CO2 levels (from greenhouse conditions of high pCO2 in the Eocene to low pCO2 ice-house conditions in the Oligocene-Miocene) and a dramatically changing ocean Mg/Ca ratio. Here we show that the most diverse, widespread, and abundant reef-building coral genus Acropora (20 morphological groups and 150 living species) has not only survived these environmental changes, but has maintained its distinct skeletal biomineralization pattern for at least 40 My: Well-preserved fossil Acropora skeletons from the Eocene, Oligocene, and Miocene show ultra-structures indistinguishable from those of extant representatives of the genus and their aragonitic skeleton Mg/Ca ratios trace the inferred ocean Mg/Ca ratio precisely since the Eocene. Therefore, among marine biogenic carbonate fossils, well-preserved acroporid skeletons represent material with very high potential for reconstruction of ancient ocean chemistry. Genomic sequencing has transformed our understanding of the evolution of scleractinian corals. However, the molecular clades defined for scleractinians are difficult to reconcile with traditional taxonomic classification based on overall skeletal morphology1–3. Instead, they have been shown to be broadly consistent with recently defined micro-morphological and ultrastructural skeletal criteria4–7. -

Sexual Reproduction of the Solitary Sunset Cup Coral Leptopsammia Pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean
Marine Biology (2005) 147: 485–495 DOI 10.1007/s00227-005-1567-z RESEARCH ARTICLE S. Goffredo Æ J. Radetic´Æ V. Airi Æ F. Zaccanti Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis Received: 16 July 2004 / Accepted: 18 December 2004 / Published online: 3 March 2005 Ó Springer-Verlag 2005 Abstract Information on the reproduction in scleractin- came indented, assuming a sickle or dome shape. We can ian solitary corals and in those living in temperate zones hypothesize that the nucleus’ migration and change of is notably scant. Leptopsammia pruvoti is a solitary coral shape may have to do with facilitating fertilization and living in the Mediterranean Sea and along Atlantic determining the future embryonic axis. During oogene- coasts from Portugal to southern England. This coral sis, oocyte diameter increased from a minimum of 20 lm lives in shaded habitats, from the surface to 70 m in during the immature stage to a maximum of 680 lm depth, reaching population densities of >17,000 indi- when mature. Embryogenesis took place in the coelen- viduals mÀ2. In this paper, we discuss the morphological teron. We did not see any evidence that even hinted at aspects of sexual reproduction in this species. In a sep- the formation of a blastocoel; embryonic development arate paper, we report the quantitative data on the an- proceeded via stereoblastulae with superficial cleavage. nual reproductive cycle and make an interspecific Gastrulation took place by delamination. Early and late comparison of reproductive traits among Dend- embryos had diameters of 204–724 lm and 290–736 lm, rophylliidae aimed at defining different reproductive respectively. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
![Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]](https://docslib.b-cdn.net/cover/7255/ornamental-fish-and-marine-invertebrates-draft-for-consultation-document-date-1037255.webp)
Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date]
Ornamental Fish and Marine Invertebrates ORNAMARI.ALL [Document Date] Health Standard Import Import Issued under the Biosecurity Act 1993 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] TITLE Import Health Standard: Ornamental Fish and Marine Invertebrates COMMENCEMENT This Import Health Standard comes into force on [Effective Date] REVOCATION This Import Health Standard revokes and replaces: Import Health Standard for Ornamental Fish and Marine Invertebrates from all countries, 20 April 2011. ISSUING AUTHORITY This Import Health Standard is issued on Dated at Wellington this ... day of ......... Howard Pharo Manager, Import and Export Animals Ministry for Primary Industries (acting under delegated authority of the Director-General) Contact for further information Ministry for Primary Industries (MPI) Regulation & Assurance Branch Animal Imports PO Box 2526 Wellington 6140 Email: [email protected] Ministry for Primary Industries Page 1 of 75 Import Health Standard: Ornamental Fish and Marine Invertebrates Draft for Consultation [Document Date] Contents Page Introduction 4 Part 1: Requirements 6 1.1 Application 6 1.2 Outcome 6 1.3 Incorporation by reference 7 1.4 Definitions 7 1.5 Harmonised system (HS) codes 7 1.6 Exporting country systems and certification 8 1.7 Diagnostic testing and treatment 8 1.8 Packaging 9 1.9 Import permit 9 1.10 The documentation that must accompany goods 9 1.11 Inspection and verification 10 1.12 Transitional facility 11 1.13 Pre-export isolation -

Journal of the Royal Society of Western Australia
Journal of the Royal Society of Western Australia ISSN 0035-922X CONTENTS Page RESEARCH PAPERS Influence of climatic gradients on metacommunities of aquatic invertebrates on granite outcrops in southern Western Australia B V Timms 125 Foraminifera from microtidal rivers with large seasonal salinity variation, southwest Western Australia D B Ostrognay & D W Haig 137 Abundance, distribution and new records of scleractinian corals at Barrow Island and Southern Montebello Islands, Pilbara (Offshore) Bioregion Z T Richards & N L Rosser 155 RESEARCH NOTES Searching for signs of bilby (Macrotis lagotis) activity in central Western Australia using observers on horseback N Burrows, J Dunlop & S Burrows 167 Hydrated body-fluid osmolality values for species of Cyclorana S J Reynolds 171 AUTHOR INDEX VOLUME 95 175 SUBJECT INDEX VOLUME 95 176 CONTENTS VOLUME 95 Volume 95 Parts 3, 4 November 2012 The Royal Society of Western Australia To promote and foster science in Western Australia Patron Her Majesty the Queen Vice-Patrons His Excellency Mr Malcolm J McCusker, AC CVO QC Governor of Western Australia Professor Lyn Beazley AO FTSE MA PhD COUNCIL 2011-2013 President P O'Brien Hon Librarian Immediate Past President L Milne Journal Manager Vice-President H Bekle Councillors Vice-President K Trinajstic A W R Bevan Hon Secretaries L Collins Council L Milne C Florides Membership J Rosser M Gallegos Proceedings W A Loneragan Hon Treasurer P O'Brien S Toby Hon Editor A E Cockbain K Wright EDITORIAL BOARD 2011-2013 Editor-in-chief K A Haskard, Data Analysis Australia A E Cockbain, South Perth P Ladd, Murdoch University Associate Editors D Laird, Murdoch University H Bekle, Notre Dame University K Trinajstic, Curtin University A Bevan, Western Australian Museum M Van Keulen, Murdoch University R Davis, Edith Cowan University K Wright, Curtin University The Royal Society of Western Australia was founded in 1914. -

CNIDARIA Corals, Medusae, Hydroids, Myxozoans
FOUR Phylum CNIDARIA corals, medusae, hydroids, myxozoans STEPHEN D. CAIRNS, LISA-ANN GERSHWIN, FRED J. BROOK, PHILIP PUGH, ELLIOT W. Dawson, OscaR OcaÑA V., WILLEM VERvooRT, GARY WILLIAMS, JEANETTE E. Watson, DENNIS M. OPREsko, PETER SCHUCHERT, P. MICHAEL HINE, DENNIS P. GORDON, HAMISH J. CAMPBELL, ANTHONY J. WRIGHT, JUAN A. SÁNCHEZ, DAPHNE G. FAUTIN his ancient phylum of mostly marine organisms is best known for its contribution to geomorphological features, forming thousands of square Tkilometres of coral reefs in warm tropical waters. Their fossil remains contribute to some limestones. Cnidarians are also significant components of the plankton, where large medusae – popularly called jellyfish – and colonial forms like Portuguese man-of-war and stringy siphonophores prey on other organisms including small fish. Some of these species are justly feared by humans for their stings, which in some cases can be fatal. Certainly, most New Zealanders will have encountered cnidarians when rambling along beaches and fossicking in rock pools where sea anemones and diminutive bushy hydroids abound. In New Zealand’s fiords and in deeper water on seamounts, black corals and branching gorgonians can form veritable trees five metres high or more. In contrast, inland inhabitants of continental landmasses who have never, or rarely, seen an ocean or visited a seashore can hardly be impressed with the Cnidaria as a phylum – freshwater cnidarians are relatively few, restricted to tiny hydras, the branching hydroid Cordylophora, and rare medusae. Worldwide, there are about 10,000 described species, with perhaps half as many again undescribed. All cnidarians have nettle cells known as nematocysts (or cnidae – from the Greek, knide, a nettle), extraordinarily complex structures that are effectively invaginated coiled tubes within a cell. -
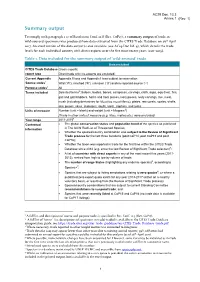
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g.