Jpet #119057 1 Title Page Characterization of Renal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phosphodiesterase 1B Knock-Out Mice Exhibit Exaggerated Locomotor Hyperactivity and DARPP-32 Phosphorylation in Response to Dopa
The Journal of Neuroscience, June 15, 2002, 22(12):5188–5197 Phosphodiesterase 1B Knock-Out Mice Exhibit Exaggerated Locomotor Hyperactivity and DARPP-32 Phosphorylation in Response to Dopamine Agonists and Display Impaired Spatial Learning Tracy M. Reed,1,3 David R. Repaske,2* Gretchen L. Snyder,4 Paul Greengard,4 and Charles V. Vorhees1* Divisions of 1Developmental Biology and 2Endocrinology, Children’s Hospital Research Foundation, Cincinnati, Ohio 45229, 3Department of Biology, College of Mount St. Joseph, Cincinnati, Ohio 45233, and 4Laboratory of Molecular and Cellular Neuroscience, Rockefeller University, New York, New York 10021 Using homologous recombination, we generated mice lack- maze spatial-learning deficits. These results indicate that en- ing phosphodiesterase-mediated (PDE1B) cyclic nucleotide- hancement of cyclic nucleotide signaling by inactivation of hydrolyzing activity. PDE1B Ϫ/Ϫ mice showed exaggerated PDE1B-mediated cyclic nucleotide hydrolysis plays a signifi- hyperactivity after acute D-methamphetamine administra- cant role in dopaminergic function through the DARPP-32 and tion. Striatal slices from PDE1B Ϫ/Ϫ mice exhibited increased related transduction pathways. levels of phospho-Thr 34 DARPP-32 and phospho-Ser 845 Key words: phosphodiesterases; DARPP-32; dopamine- GluR1 after dopamine D1 receptor agonist or forskolin stimu- stimulated locomotor activity; spatial learning and memory; lation. PDE1B Ϫ/Ϫ and PDE1B ϩ/Ϫ mice demonstrated Morris Morris water maze; methamphetamine; SKF81297; forskolin Calcium/calmodulin-dependent phosphodiesterases (CaM- (CaMKII) and calcineurin and have the potential to activate PDEs) are members of one of 11 families of PDEs (Soderling et CaM-PDEs. Dopamine D1 or D2 receptor activation leads to al., 1999;Yuasa et al., 2001) and comprise the only family that acts adenylyl cyclase activation or inhibition, respectively (Traficante ϩ as a potential point of interaction between the Ca 2 and cyclic et al., 1976; Monsma et al., 1990; Cunningham and Kelley, 1993; nucleotide signaling pathways. -

N-Glycan Trimming in the ER and Calnexin/Calreticulin Cycle
Neurotransmitter receptorsGABA and A postsynapticreceptor activation signal transmission Ligand-gated ion channel transport GABAGABA Areceptor receptor alpha-5 alpha-1/beta-1/gamma-2 subunit GABA A receptor alpha-2/beta-2/gamma-2GABA receptor alpha-4 subunit GABAGABA receptor A receptor beta-3 subunitalpha-6/beta-2/gamma-2 GABA-AGABA receptor; A receptor alpha-1/beta-2/gamma-2GABA receptoralpha-3/beta-2/gamma-2 alpha-3 subunit GABA-A GABAreceptor; receptor benzodiazepine alpha-6 subunit site GABA-AGABA-A receptor; receptor; GABA-A anion site channel (alpha1/beta2 interface) GABA-A receptor;GABA alpha-6/beta-3/gamma-2 receptor beta-2 subunit GABAGABA receptorGABA-A receptor alpha-2receptor; alpha-1 subunit agonist subunit GABA site Serotonin 3a (5-HT3a) receptor GABA receptorGABA-C rho-1 subunitreceptor GlycineSerotonin receptor subunit3 (5-HT3) alpha-1 receptor GABA receptor rho-2 subunit GlycineGlycine receptor receptor subunit subunit alpha-2 alpha-3 Ca2+ activated K+ channels Metabolism of ingested SeMet, Sec, MeSec into H2Se SmallIntermediateSmall conductance conductance conductance calcium-activated calcium-activated calcium-activated potassium potassium potassiumchannel channel protein channel protein 2 protein 1 4 Small conductance calcium-activatedCalcium-activated potassium potassium channel alpha/beta channel 1 protein 3 Calcium-activated potassiumHistamine channel subunit alpha-1 N-methyltransferase Neuraminidase Pyrimidine biosynthesis Nicotinamide N-methyltransferase Adenosylhomocysteinase PolymerasePolymeraseHistidine basic -

Regulation of Calmodulin-Stimulated Cyclic Nucleotide Phosphodiesterase (PDE1): Review
95-105 5/6/06 13:44 Page 95 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 18: 95-105, 2006 95 Regulation of calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1): Review RAJENDRA K. SHARMA, SHANKAR B. DAS, ASHAKUMARY LAKSHMIKUTTYAMMA, PONNIAH SELVAKUMAR and ANURAAG SHRIVASTAV Department of Pathology and Laboratory Medicine, College of Medicine, University of Saskatchewan, Cancer Research Division, Saskatchewan Cancer Agency, 20 Campus Drive, Saskatoon SK S7N 4H4, Canada Received January 16, 2006; Accepted March 13, 2006 Abstract. The response of living cells to change in cell 6. Differential inhibition of PDE1 isozymes and its environment depends on the action of second messenger therapeutic applications molecules. The two second messenger molecules cAMP and 7. Role of proteolysis in regulating PDE1A2 Ca2+ regulate a large number of eukaryotic cellular events. 8. Role of PDE1A1 in ischemic-reperfused heart Calmodulin-stimulated cyclic nucleotide phosphodiesterase 9. Conclusion (PDE1) is one of the key enzymes involved in the complex interaction between cAMP and Ca2+ second messenger systems. Some PDE1 isozymes have similar kinetic and 1. Introduction immunological properties but are differentially regulated by Ca2+ and calmodulin. Accumulating evidence suggests that the A variety of cellular activities are regulated through mech- activity of PDE1 is selectively regulated by cross-talk between anisms controlling the level of cyclic nucleotides. These Ca2+ and cAMP signalling pathways. These isozymes are mechanisms include synthesis, degradation, efflux and seque- also further distinguished by various pharmacological agents. stration of cyclic adenosine 3':5'-monophosphate (cAMP) and We have demonstrated a potentially novel regulation of PDE1 cyclic guanosine 3':5'- monophosphate (cGMP) within the by calpain. -

Analyses of PDE-Regulated Phosphoproteomes Reveal Unique and Specific Camp-Signaling Modules in T Cells
Analyses of PDE-regulated phosphoproteomes reveal unique and specific cAMP-signaling modules in T cells Michael-Claude G. Beltejara, Ho-Tak Laua, Martin G. Golkowskia, Shao-En Onga, and Joseph A. Beavoa,1 aDepartment of Pharmacology, University of Washington, Seattle, WA 98195 Contributed by Joseph A. Beavo, May 28, 2017 (sent for review March 10, 2017; reviewed by Paul M. Epstein, Donald H. Maurice, and Kjetil Tasken) Specific functions for different cyclic nucleotide phosphodiester- to bias T-helper polarization toward Th2, Treg, or Th17 pheno- ases (PDEs) have not yet been identified in most cell types. types (13, 14). In a few cases increased cAMP may even potentiate Conventional approaches to study PDE function typically rely on the T-cell activation signal (15), particularly at early stages of measurements of global cAMP, general increases in cAMP- activation. Recent MS-based proteomic studies have been useful dependent protein kinase (PKA), or the activity of exchange in characterizing changes in the phosphoproteome of T cells under protein activated by cAMP (EPAC). Although newer approaches various stimuli such as T-cell receptor stimulation (16), prosta- using subcellularly targeted FRET reporter sensors have helped glandin signaling (17), and oxidative stress (18), so much of the define more compartmentalized regulation of cAMP, PKA, and total Jurkat phosphoproteome is known. Until now, however, no EPAC, they have limited ability to link this regulation to down- information on the regulation of phosphopeptides by PDEs has stream effector molecules and biological functions. To address this been available in these cells. problem, we have begun to use an unbiased mass spectrometry- Inhibitors of cAMP PDEs are useful tools to study PKA/EPAC- based approach coupled with treatment using PDE isozyme- mediated signaling, and selective inhibitors for each of the 11 PDE – selective inhibitors to characterize the phosphoproteomes of the families have been developed (19 21). -

Phosphodiesterase (PDE)
Phosphodiesterase (PDE) Phosphodiesterase (PDE) is any enzyme that breaks a phosphodiester bond. Usually, people speaking of phosphodiesterase are referring to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases, as well as numerous less-well-characterized small-molecule phosphodiesterases. The cyclic nucleotide phosphodiesterases comprise a group of enzymes that degrade the phosphodiester bond in the second messenger molecules cAMP and cGMP. They regulate the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. PDEs are therefore important regulators ofsignal transduction mediated by these second messenger molecules. www.MedChemExpress.com 1 Phosphodiesterase (PDE) Inhibitors, Activators & Modulators (+)-Medioresinol Di-O-β-D-glucopyranoside (R)-(-)-Rolipram Cat. No.: HY-N8209 ((R)-Rolipram; (-)-Rolipram) Cat. No.: HY-16900A (+)-Medioresinol Di-O-β-D-glucopyranoside is a (R)-(-)-Rolipram is the R-enantiomer of Rolipram. lignan glucoside with strong inhibitory activity Rolipram is a selective inhibitor of of 3', 5'-cyclic monophosphate (cyclic AMP) phosphodiesterases PDE4 with IC50 of 3 nM, 130 nM phosphodiesterase. and 240 nM for PDE4A, PDE4B, and PDE4D, respectively. Purity: >98% Purity: 99.91% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 1 mg, 5 mg Size: 10 mM × 1 mL, 10 mg, 50 mg (R)-DNMDP (S)-(+)-Rolipram Cat. No.: HY-122751 ((+)-Rolipram; (S)-Rolipram) Cat. No.: HY-B0392 (R)-DNMDP is a potent and selective cancer cell (S)-(+)-Rolipram ((+)-Rolipram) is a cyclic cytotoxic agent. (R)-DNMDP, the R-form of DNMDP, AMP(cAMP)-specific phosphodiesterase (PDE) binds PDE3A directly. -
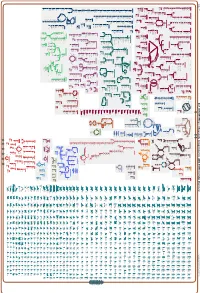
Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_003855395Cyc: Shewanella livingstonensis LMG 19866 Cellular Overview Connections between pathways are omitted for legibility. -

The Clinical Biochemistry of 5'-Nucleotidase*
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 20, No. 2 Copyright © 1990, Institute for Clinical Science, Inc. The Clinical Biochemistry of 5'-Nucleotidase* F. WILLIAM SUNDERMAN JR., M.D. Departments of Laboratory Medicine and Pharmacology, University of Connecticut Medical School, Farmington, CT 06032 ABSTRACT This review delineates the subcellular distribution, biochemical charac teristics, and metabolic functions of 5'-nucleotidase (5'NT), summarizes the analytical biochemistry of 5'NT, and assesses the clinical significance of5'NT determinations in body fluids, cells, and tissues. Salient aspects of the clinical biochemistry of 5'NT, discussed herein, are as follows: (A) Serum 5'NT activity is generally elevated in hepatobiliary diseases, espe cially with intrahepatic obstruction, but, unlike serum alkaline phospha tase, serum 5'NT activity is not increased in infancy, childhood, preg nancy, or osteoblastic disorders. (B) In cancer patients, elevated serum 5'NT activity does not always indicate hepatobiliary involvement; in some cases, 5'NT may be released into serum from the primary tumor or local metastases. (C) Genetic deficiency of erythrocyte pyrimidine 5'NT activity is a common cause of hereditary non-spherocytic hemolytic anemia. (D) Acquired deficiency of erythrocyte pyrimidine 5'NT activity occurs in patients with P-thalassemia and lead poisoning. (E) 5'NT activity is low in circulating monocytes, increases markedly upon their differentiation to tissue macrophages, and subsequently diminishes during macrophage activation. (F) Lymphocyte ecto-5'NT activity, a plasma membrane marker of cell maturation, is generally low in immunodeficiency states, and undergoes characteristic changes in patients with certain lymphomas and leukemias. Introduction ribonucleosides and inorganic phos phate. -

Poly (I)-Poly (C)/Oligo (Dt)-Cellulosei... Sidney Pestka, James Mcinnes
Contents Vol. 72, No. 10 October 1975 INFORMATION TO CONTRIBUTORS ..............................iv...........................I..................... i-i AUTfHOR INDEX ................................................................................................. 4190 Physical Sciences APPLIED Adiabatic evolution of plasma equilibrium (bifurcation/isolation/weak solution/generalized differential MATHEMATICS equation/Tokamak) ............................................ H. Grad, P.N. Hu, and D. C. Stevens 3789-3793 CHEMISTRY Theoretical studies of metal-phosphate interactions: Interaction of Li+, Na+, K+, Be++, Mg++, and Ca++ with H2PO4- and (CH30)2PO2-: Implications for nucleic acid solvation (metal binding/phos- phate complexes/molecular orbital theory) ................Dennis S. Marynick and Henry F. Schaefer III 3794-3798 Maximum-valence radii of transition metals (covalent radii/enneacovalence) ............... Linus Pauling 3799-3801 Model of protein folding: Inclusion of short-, medium-, and long-range interactions (mechanism offolding/ contact free energies/range of interactions/Monte Carlo) ........... Seiji Tanaka and HaroldA. Scheraga 3802-3806 Matrix method for fhActuations and noise in kinetic systems (cross correlation function/noise power spectrum matrix/relaxation matrix/biochemical reaction kinetics/muscle contraction) .Yi-der Chen 3807-3811 MATHEMATICS Point estimates for probability moments (approximate methods/finite differences/numerical methods/prob- abilities) .......................... - ;Emilio Rosenblueth 3812-3814 -
Generate Metabolic Map Poster
Authors: Zheng Zhao, Delft University of Technology Marcel A. van den Broek, Delft University of Technology S. Aljoscha Wahl, Delft University of Technology Wilbert H. Heijne, DSM Biotechnology Center Roel A. Bovenberg, DSM Biotechnology Center Joseph J. Heijnen, Delft University of Technology An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Marco A. van den Berg, DSM Biotechnology Center Peter J.T. Verheijen, Delft University of Technology Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. PchrCyc: Penicillium rubens Wisconsin 54-1255 Cellular Overview Connections between pathways are omitted for legibility. Liang Wu, DSM Biotechnology Center Walter M. van Gulik, Delft University of Technology L-quinate phosphate a sugar a sugar a sugar a sugar multidrug multidrug a dicarboxylate phosphate a proteinogenic 2+ 2+ + met met nicotinate Mg Mg a cation a cation K + L-fucose L-fucose L-quinate L-quinate L-quinate ammonium UDP ammonium ammonium H O pro met amino acid a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar K oxaloacetate L-carnitine L-carnitine L-carnitine 2 phosphate quinic acid brain-specific hypothetical hypothetical hypothetical hypothetical -

Phosphodiesterases Expression During Murine Cardiac Development
International Journal of Molecular Sciences Article Phosphodiesterases Expression during Murine Cardiac Development Thays Maria da Conceição Silva Carvalho 1,†, Silvia Cardarelli 1,†, Mauro Giorgi 2, Andrea Lenzi 3, Andrea M. Isidori 3 and Fabio Naro 1,* 1 Department of Anatomical, Histological, Forensic and Orthopedic Sciences, Sapienza University, 00161 Rome, Italy; [email protected] (T.M.d.C.S.C.); [email protected] (S.C.) 2 Department of Biology and Biotechnology “C. Darwin”, Sapienza University, 00185 Rome, Italy; [email protected] 3 Department of Experimental Medicine, Sapienza University, 00161 Rome, Italy; [email protected] (A.L.); [email protected] (A.M.I.) * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: 30-50 cyclic nucleotide phosphodiesterases (PDEs) are a large family of enzymes playing a fundamental role in the control of intracellular levels of cAMP and cGMP. Emerging evidence suggested an important role of phosphodiesterases in heart formation, but little is known about the expression of phosphodiesterases during cardiac development. In the present study, the pattern of expression and enzymatic activity of phosphodiesterases was investigated at different stages of heart formation. C57BL/6 mice were mated and embryos were collected from 14.5 to 18.5 days of development. Data obtained by qRT-PCR and Western blot analysis showed that seven different isoforms are expressed during heart development, and PDE1C, PDE2A, PDE4D, PDE5A and PDE8A Citation: Carvalho, T.M.d.C.S.; are modulated from E14.5 to E18.5. In heart homogenates, the total cAMP and cGMP hydrolytic Cardarelli, S.; Giorgi, M.; Lenzi, A.; activity is constant at the evaluated times, and PDE4 accounts for the majority of the cAMP hydrolyz- Isidori, A.M.; Naro, F. -

Supplementary. Table S1 a Total of Degs Detected in This Study (Gm) No
Supplementary. Table S1 A total of DEGs detected in this study (Gm) No. genename significance in annotation 1 At1g01020 D2 ARV1__expressed protein, similar to hypothetical protein DDB0188786 [Dictyostelium discoideum] (GB:EAL62332.1); contains InterPro domain Arv1-like protein (InterPro:IPR007290) 2 At1g01100 D2 60S acidic ribosomal protein P1 (RPP1A), similar to 60S ACIDIC RIBOSOMAL PROTEIN P1 GB:O23095 from (Arabidopsis thaliana) 3 At1g01120 D2, Dm KCS1__fatty acid elongase 3-ketoacyl-CoA synthase 1 (KCS1), nearly identical to GB:AAC99312 GI:4091810 from (Arabidopsis thaliana) 4 At1g01160 D1, D2, Dm GIF2__SSXT protein-related / transcription co-activator-related, similar to SYT/SSX4 fusion protein (GI:11127695) (Homo sapiens); supporting cDNA gi:21539891:gb:AY102640.1:; contains Pfam profile PF05030: SSXT protein (N-terminal region) 5 At1g01170 D2 ozone-responsive stress-related protein, putative, similar to stress-related ozone-induced protein AtOZI1 (GI:790583) (Arabidopsis thaliana); contains 1 predicted transmembrane domain; 6 At1g01240 D1, D2, Dm expressed protein 7 At1g01300 D2, Dm aspartyl protease family protein, contains Pfam domain, PF00026: eukaryotic aspartyl protease 8 At1g01320 D2 tetratricopeptide repeat (TPR)-containing protein, low similarity to SP:P46825 Kinesin light chain (KLC) {Loligo pealeii}; contains Pfam profile PF00515: TPR Domain 9 At1g01430 D2, Dm expressed protein, similar to hypothetical protein GB:CAB80917 GI:7267605 from (Arabidopsis thaliana) 10 At1g01470 D1, D2, Dm LEA14_LSR3__late embryogenesis abundant -

Adaptation of Camp Signaling System in SH-SY5Y Neuroblastoma Cells Following Expression of a Constitutively Active Stimulatory G Protein Alpha, Q227L Gsα
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 33, No. 1, 37-45, March 2001 Adaptation of cAMP signaling system in SH-SY5Y neuroblastoma cells following expression of a constitutively active stimulatory G protein alpha, Q227L Gsα Ik-Soon Jang1 and Yong-Sung Juhnn1,2 cAMP system adapts at the post-receptor level to a sustained activation of the system by differential ex- 1 Department of Biochemistry and Cancer Research Institute, pression of the isoforms of AC, PDE, and PKA in SH- Seoul National University College of Medicine, Seoul 110-799, SY5Y neuroblastoma. We also showed that an in- Korea crease in cellular cAMP concentration might medi- 2 Corresponding author: Tel, +82-2-740-8247; Fax, +82-2-744-4534; ate the observed changes in the cAMP system. E-mail, [email protected] Keywords: adenylate cyclase, cAMP-dependent protein Accepted 23 March, 2000 kinase, 3’,5’-cyclic-nucleotide phosphodiesterase, Pro- tein isoforms, reverse transcriptase polymerase chain Abbreviations: AC, adenylate cyclase; ACI, AC isoform I; dbcAMP, reaction dibutyryl cAMP; GAPDH, glyceraldehyde-3-phosphate dehydroge- nase; Gsα, alpha subunit of stimulatory G protein; PDE, cyclic nucleotide phosphodiesterase; PKA, cAMP-dependent protein Introduction kinase; RT-PCR, reverse transcription-polymerase chain reaction Signal transducing heterotrimeric GTP-binding proteins (G protein) relay signals from activated receptors to intracellular effectors to elicit cellular responses (Gilman, Abstract 1987). Binding of signaling molecules such as hormones Heterotrimeric GTP-binding proteins (G protein) are and neurotransmitters to the receptors makes the receptor known to participate in the transduction of signals undergo conformational change. This conformational from ligand activated receptors to effector mole- change leads to activation of G proteins by promoting cules to elicit cellular responses.