Gastropoda: Muricidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Shell Classification – Using Family Plates
Shell Classification USING FAMILY PLATES YEAR SEVEN STUDENTS Introduction In the following activity you and your class can use the same techniques as Queensland Museum The Queensland Museum Network has about scientists to classify organisms. 2.5 million biological specimens, and these items form the Biodiversity collections. Most specimens are from Activity: Identifying Queensland shells by family. Queensland’s terrestrial and marine provinces, but These 20 plates show common Queensland shells some are from adjacent Indo-Pacific regions. A smaller from 38 different families, and can be used for a range number of exotic species have also been acquired for of activities both in and outside the classroom. comparative purposes. The collection steadily grows Possible uses of this resource include: as our inventory of the region’s natural resources becomes more comprehensive. • students finding shells and identifying what family they belong to This collection helps scientists: • students determining what features shells in each • identify and name species family share • understand biodiversity in Australia and around • students comparing families to see how they differ. the world All shells shown on the following plates are from the • study evolution, connectivity and dispersal Queensland Museum Biodiversity Collection. throughout the Indo-Pacific • keep track of invasive and exotic species. Many of the scientists who work at the Museum specialise in taxonomy, the science of describing and naming species. In fact, Queensland Museum scientists -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -

The Recent Molluscan Marine Fauna of the Islas Galápagos
THE FESTIVUS ISSN 0738-9388 A publication of the San Diego Shell Club Volume XXIX December 4, 1997 Supplement The Recent Molluscan Marine Fauna of the Islas Galapagos Kirstie L. Kaiser Vol. XXIX: Supplement THE FESTIVUS Page i THE RECENT MOLLUSCAN MARINE FAUNA OF THE ISLAS GALApAGOS KIRSTIE L. KAISER Museum Associate, Los Angeles County Museum of Natural History, Los Angeles, California 90007, USA 4 December 1997 SiL jo Cover: Adapted from a painting by John Chancellor - H.M.S. Beagle in the Galapagos. “This reproduction is gifi from a Fine Art Limited Edition published by Alexander Gallery Publications Limited, Bristol, England.” Anon, QU Lf a - ‘S” / ^ ^ 1 Vol. XXIX Supplement THE FESTIVUS Page iii TABLE OF CONTENTS INTRODUCTION 1 MATERIALS AND METHODS 1 DISCUSSION 2 RESULTS 2 Table 1: Deep-Water Species 3 Table 2: Additions to the verified species list of Finet (1994b) 4 Table 3: Species listed as endemic by Finet (1994b) which are no longer restricted to the Galapagos .... 6 Table 4: Summary of annotated checklist of Galapagan mollusks 6 ACKNOWLEDGMENTS 6 LITERATURE CITED 7 APPENDIX 1: ANNOTATED CHECKLIST OF GALAPAGAN MOLLUSKS 17 APPENDIX 2: REJECTED SPECIES 47 INDEX TO TAXA 57 Vol. XXIX: Supplement THE FESTIVUS Page 1 THE RECENT MOLLUSCAN MARINE EAUNA OE THE ISLAS GALAPAGOS KIRSTIE L. KAISER' Museum Associate, Los Angeles County Museum of Natural History, Los Angeles, California 90007, USA Introduction marine mollusks (Appendix 2). The first list includes The marine mollusks of the Galapagos are of additional earlier citations, recent reported citings, interest to those who study eastern Pacific mollusks, taxonomic changes and confirmations of 31 species particularly because the Archipelago is far enough from previously listed as doubtful. -

(Bruguiere, 1789) (Gastropoda, Muricidae) in Fjords and Channels of Southern Chile Revista Chilena De Historia Natural, Vol
Revista Chilena de Historia Natural ISSN: 0716-078X [email protected] Sociedad de Biología de Chile Chile MOLINET, CARLOS; ARÉVALO, ALEJANDRA; GONZÁLEZ, MARÍA TERESA; MORENO, CARLOS A.; ARATA, JAVIER; NIKLITSCHEK, EDWIN Patterns of larval distribution and settlement of Concholepas concholepas (Bruguiere, 1789) (Gastropoda, Muricidae) in fjords and channels of southern Chile Revista Chilena de Historia Natural, vol. 78, núm. 3, 2005, pp. 409-423 Sociedad de Biología de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=369944275005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LARVAL ECOLOGY OF C. CONCHOLEPAS IN SOUTHERNRevista CHILE Chilena de Historia Natural409 78: 409-423, 2005 Patterns of larval distribution and settlement of Concholepas concholepas (Bruguiere, 1789) (Gastropoda, Muricidae) in fjords and channels of southern Chile Patrones de distribución de larvas y asentamiento de Concholepas concholepas (Bruguiere, 1789) (Gastropoda, Muricidae) en fiordos y canales del sur de Chile CARLOS MOLINET1,3, ALEJANDRA ARÉVALO1, MARÍA TERESA GONZÁLEZ2, CARLOS A. MORENO2, JAVIER ARATA2 & EDWIN NIKLITSCHEK1 1Centro Universitario de la Trapananda e 2Instituto de Ecología y Evolución, 3Instituto de Acuicultura, Universidad Austral de Chile, Casilla 567, Valdivia, Chile; e-mail: [email protected] ABSTRACT The distribution of Concholepas concholepas (Mollusca, Gastropoda, Muricidae) is limited to the coasts of Chile and southern Peru. Almost all studies of this gastropod have been carried out in open coastal systems, rather than the fjords and channels of southern Chile, despite the fact that this area represents ca. -

Business Plan for a Snails Farm in the Republic of Moldova
Business Plan for a Snails Farm in the Republic of Moldova M.Ec. Tincuța Chircu Master thesis 2017 TBU in Zlín, Faculty of Management and Economics 2 TBU in Zlín, Faculty of Management and Economics 3 TBU in Zlín, Faculty of Management and Economics 4 TBU in Zlín, Faculty of Management and Economics 5 ABSTRAKT Cílem projektu je tvorba business plánu šnečí farmy v Moldávii se zaměřující se na export šnečích produktů na evropský trh. Přestože je poptávka po tomto produktu na místním trhu téměř nulová, v některých evropských zemích nejsou šnečí produkty i přes poptávku dostupné. Většina produktů bude určena k exportu, budou však nabízeny i na moldavském trhu. Teoretická část popisuje jednotlivé části business plánu, zejména z pohledu tzv. plátna business modelu, typy šneků a životního prostředí, analytické nástroje pro podnikání a legislativní požadavky. Druhá část analyzuje šnečí trh z globálního hlediska, detailněji je rozebrán evropský trh. Třetí část tvoří samotný business plán, zahrnující veškeré využité kroky a aktivity, časový rozvrh a analýzu rizik. Klíčová slova: business plán, Canvas Business Model, Snails farming, SWOT analýza, PESTEL analýza, Porterova analýza ABSTRACT The aim of this project is to create a business plan for a snails farm in the Republic of Moldova, with the purpose the export the product to the European market. Although the demand is almost inexistent on the local market, there is a need of snail products that is not covered in some European countries. Most of the products will be intended for export, but there will also be offers for the Moldovan market. The theoretical part describes the components of a business plan, with a special focus on Canvas Business Model, the snails types and environment, the analytical tools for a busi- ness and the legal requirements. -
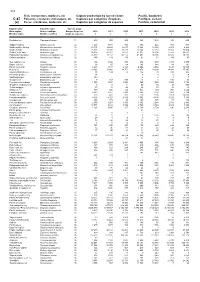
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

Mollusks of Manuel Antonio National Park, Pacific Costa Rica
Rev. Biol. Trop. 49. Supl. 2: 25-36, 2001 www.rbt.ac.cr, www.ucr.ac.cr Mollusks of Manuel Antonio National Park, Pacific Costa Rica Samuel Willis 1 and Jorge Cortés 2-3 1140 East Middle Street, Gettysburg, Pennsylvania 17325, USA. 2Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Universidad de Costa Rica, 2060 San José, Costa Rica. FAX: (506) 207-3280. E-mail: [email protected] 3Escuela de Biología, Universidad de Costa Rica, 2060 San José, Costa Rica. (Received 14-VII-2000. Corrected 23-III-2001. Accepted 11-V-2001) Abstract: The mollusks in Manuel Antonio National Park on the central section of the Pacific coast of Costa Rica were studied along thirty-six transects done perpendicular to the shore, and by random sampling of subtidal environments, beaches and mangrove forest. Seventy-four species of mollusks belonging to three classes and 40 families were found: 63 gastropods, 9 bivalves and 2 chitons, during this study in 1995. Of these, 16 species were found only as empty shells (11) or inhabited by hermit crabs (5). Forty-eight species were found at only one locality. Half the species were found at one site, Puerto Escondido. The most diverse habitat was the low rocky intertidal zone. Nodilittorina modesta was present in 34 transects and Nerita scabricosta in 30. Nodilittorina aspera had the highest density of mollusks in the transects. Only four transects did not clustered into the four main groups. The species composition of one cluster of transects is associated with a boulder substrate, while another cluster of transects associates with site. -

Cariotipos De Los Caracoles De Tinte Plicopurpura Pansa Y Plicopurpura Columellaris (Gastropoda: Muricidae)
Cariotipos de los caracoles de tinte Plicopurpura pansa y Plicopurpura columellaris (Gastropoda: Muricidae) Lenin Arias-Rodriguez1,3, Juan P. González-Hermoso2, Horacio Fletes-Regalado2, Luz Estela Rodríguez-Ibarra1 & Gabriela Del Valle Pignataro1 1 Centro de Investigación en Alimentación y Desarrollo, A.C. Unidad, Mazatlán, Sábalo-Cerritos S/N Estero del Yugo, A.P. 711, Mazatlán, Sinaloa, México. Tel. (669) 989-87-00. Fax (669) 989-87-01; [email protected] 2 Universidad Autónoma de Nayarit, Facultad de Ingeniería Pesquera, Bahia de Matanchan, Km 12, Carretera los Cocos, A.P. 10, San Blas Nayarit, Mexico. Tel/Fax. (323) 31-21-20. 3 División Académica de Ciencias Biológicas, UJAT. Carretera Villahermosa-Cárdenas Km 0.5 S/N. Entronque a Bosques de Saloya. C.P. 86150. Tel. (993) 354-4308. Villahermosa, Tabasco, México. Recibido 04-III-2006. Corregido 11-XII-2006. Aceptado 14-V-2007. Abstract: Karyotypes of the purple snails Plicopurpura pansa and Plicopurpura columellaris (Gastropoda: Muricidae). The karyotypes of the purple snails Plicopurpura pansa (Gould, 1853) and P. columellaris (Lamarck, 1816) were established from 17 and 13 adults, respectively; and from eight capsules with embryos of P. pansa. In P. pansa were counted 59 mitotic fields in the adults and 127 in embryos; and 118 fields in P. columellaris. Chromosome numbers from 30 to 42 were observed in both species. Such a variation was notori- ous in each sample and there was no evidence of any relationship with tissue (gill, muscle and stomach). Both species has a typical modal number of 2n=36 chromosomes. Five good quality chromosome spreads were selected from adults of each species to assemble the karyotype. -

Imposex in Plicopurpura Pansa (Neogastropoda: Thaididae)
Available online at www.sciencedirect.com View metadata, citation and similar papers at core.ac.uk brought to you by CORE Revista Mexicana de Biodiversidad provided by Elsevier - Publisher Connector Revista Mexicana de Biodiversidad 86 (2015) 531–534 www.ib.unam.mx/revista/ Research note Imposex in Plicopurpura pansa (Neogastropoda: Thaididae) in Nayarit and Sinaloa, Mexico Imposex en Plicopurpura pansa (Neogastropoda: Thaididae) en Nayarit and Sinaloa, México Delia Domínguez-Ojeda a, Olga Araceli Patrón-Soberano b, José Trinidad Nieto-Navarro a,∗, María de Lourdes Robledo-Marenco c, Jesús Bernardino Velázquez-Fernández c a Escuela Nacional de Ingeniería Pesquera, Universidad Autónoma de Nayarit, Bahía de Matanchén km 12, carretera a Los Cocos, 63740, San Blas, Nayarit, Mexico b División de Biología Molecular, Instituto Potosino de Investigación Científica y Tecnológica, Camino a la presa de San José, Núm. 2055, Lomas 4ta, Sección, 78216 San Luís Potosí, Mexico c Laboratorio de Contaminación y Toxicología Ambiental, Universidad Autónoma de Nayarit, Ciudad de la Cultura Amado Nervo, S/N, Los Fresnos, 63155, Tepic, Nayarit, Mexico Received 25 June 2014; accepted 10 February 2015 Available online 26 May 2015 Abstract Imposex is the development of male features in female prosobranch gastropods, caused by organotin compounds. In the Mexican Pacific coast, imposex was observed in Plicopurpura pansa. This snail has been used by indigenous people to dye cotton and traditional fabric clothing. During 2010 and 2011, 5 habitats were visited along the coastline of Nayarit and Sinaloa, Mexico. At low tide, 675 snails were collected. Shell length, sex ratio and imposex incidence were measured. Imposex incidences were higher in the samples collected near harbor areas. -

Download Download
ISJ 11: 204-212, 2014 ISSN 1824-307X RESEARCH REPORT Exposure to tributyltin chloride induces penis and vas deferens development and increases RXR expression in females of the purple snail (Plicopurpura pansa) D Domínguez-Ojeda1, AE Rojas-García2, ML Robledo-Marenco2, BS Barrón-Vivanco2, IM Medina-Díaz2 1Escuela Nacional de Ingeniería Pesquera, Universidad Autónoma de Nayarit, Bahía de Matanchén Km. 12, San Blas, 63740 Nayarit, México 2Laboratorio de Contaminación y Toxicología Ambiental, Universidad Autónoma de Nayarit, Av. de la Cultura s/n, Col. Los Fresnos, 63155 Tepic, Nayarit, México Accepted June 26, 2014 Abstract Tributyltin (TBT) and its derivatives are widely used as antifouling paints for ships, resulting in their being released into the marine environment. Aquatic invertebrates, particularly marine gastropods, are extremely sensitive to TBT and undergo changes in the imposition of male secondary sex characteristics in response to exposure. This study aimed to evaluate the development of imposex and the expression of the retinoid X receptor (RXR) in tissues of Plicopurpura pansa (males and females) exposed to tributyltin chloride (TBTCl). The histological results showed a penis-like structure in imposexed female and an undeveloped vas deferens that lacked circular muscular layers. TBTCl treatment increased the messenger RNA (mRNA) of RXR in females with imposex. The highest level of mRNA RXR was found in the digestive gland and penis-forming area in females under in vivo exposure compared with control females. These results indicate that TBTCl modulates mRNA levels of RXR in females. mRNA RXR in imposex females and females exposed to TBTCl only was similar to that of males, indicating that RXR might contribute to the development of imposex. -

Main Courses Appetisers Soups Salads Desserts
APPETISERS MAIN COURSES Beef carpaccio with cherry tomatoes and shallot onions mari- Beefsteak served in a pan with boiled roasted La Ratte nated in beetroot juice, drizzled with honey-balsamic sauce potatoes, fried vegetables, seasoned with red wine and 12.00 € green pepper sauce or chilli butter 26.00 € Recommended wine Abadia de San Quirce Crianza Giant freshwater prawn tails, pumpkin and citrus fruit mousse, home-made mayonnaise (with squid ink), snail caviar Duck fillet served with red Quinoa and pearled barley 12.00 € risotto, caramelized fig, orange and kumquat sauce 18.00 € Veal and duck liver pâté with truffles, seasoned with white Recommended wine Johannishof Charta Riesling wine jelly, served with a pear poached in orange juice and a bread toast Halibut fillet accompanied by Bulgur wheat, green 11.00 € asparagus, tomato baked with pesto and crayfish tail sauce 19.00 € Roasted duck liver served on a toast, seasoned with blue Recommended wine Principi di Butera Chardonnay IGT onion and raspberry jam 18.00 € Octopus served with butter-fried cherry tomatoes and boiled fried potatoes 21.00 € SOUPS Recommended wine Clarendelle Blanc Bordeaux AOCIGT Vegetable soup with homemade dumplings stuffed with duck meat Lithuanian Special 6.00 € Veal loin steak with bone served with potato, roasted garlic and crispy bacon mousse, caramelized carrots, red wine, truffle and black currant sauce Beetroot soup with “skilandis” and boletus mushrooms 24.00 € 5.00 € Recommended wine Paolo Leo Primitivo di Manduria Fish soup with halibut fillet and