Conditional Ablation of Cerebellar Astrocytes in Postnatal Transgenic Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2
667 The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2. The Cerebellar Hemispheres Gary A. Press 1 Thin (5-mm) sagittal high-field (1 .5-T) MR images of the cerebellar hemispheres James Murakami2 display (1) the superior, middle, and inferior cerebellar peduncles; (2) the primary white Eric Courchesne2 matter branches to the hemispheric lobules including the central, anterior, and posterior Dean P. Berthoty1 quadrangular, superior and inferior semilunar, gracile, biventer, tonsil, and flocculus; Marjorie Grafe3 and (3) several finer secondary white-matter branches to individual folia within the lobules. Surface features of the hemispheres including the deeper fissures (e.g., hori Clayton A. Wiley3 1 zontal, posterolateral, inferior posterior, and inferior anterior) and shallower sulci are John R. Hesselink best delineated on T1-weighted (short TRfshort TE) and T2-weighted (long TR/Iong TE) sequences, which provide greatest contrast between CSF and parenchyma. Correlation of MR studies of three brain specimens and 11 normal volunteers with microtome sections of the anatomic specimens provides criteria for identifying confidently these structures on routine clinical MR. MR should be useful in identifying, localizing, and quantifying cerebellar disease in patients with clinical deficits. The major anatomic structures of the cerebellar vermis are described in a companion article [1). This communication discusses the topographic relationships of the cerebellar hemispheres as seen in the sagittal plane and correlates microtome sections with MR images. Materials, Subjects, and Methods The preparation of the anatomic specimens, MR equipment, specimen and normal volunteer scanning protocols, methods of identifying specific anatomic structures, and system of This article appears in the JulyI August 1989 issue of AJNR and the October 1989 issue of anatomic nomenclature are described in our companion article [1]. -

Introduction
Cambridge University Press 978-1-316-64693-9 — The Brain and Behavior 4th Edition Excerpt More Information Chapter1 Introduction Introduction Theneuraxisinthehumanrunsasanimaginary straight line through the center of the spinal cord Human behavior is a direct reflection of the anatomy and brainstem (Figure 1.1). At the level of the junc- and physiology of the central nervous system. The goal tion of the midbrain and diencephalon, however, the of the behavioral neuroscientist is to uncover the neu- neuraxis changes orientation and extends from the roanatomical substrates of behavior. Complex mental occipital pole to the frontal pole (Figure 1.1). processes are represented in the brain by their elemen- The neuraxis located above the midbrain is the neur- tary components. Elaborate mental functions consist of axis of the cerebrum and is sometimes called the subfunctions and are constructed from both serial and horizontal neuraxis. A cross-section taken perpendi- parallel interconnections of several brain regions. cular to the horizontal neuraxis is called a coronal An introduction to the nervous system covers general (frontal) section. terminology and the ventricular system. With regard to the neuraxis of the spinal cord and brainstem: Major Subdivisions • Dorsal (posterior) means toward the back. The nervous system is divided anatomically into the • Ventral (anterior) means toward the abdomen. central nervous system (CNS) and the peripheral ner- • Rostral means toward the nose. vous system (PNS). • Caudal means toward the tail. • The CNS is made up of the brain and spinal cord. • The sagittal (midsagittal) plane is the vertical • The PNS consists of the cranial nerves and spinal plane that passes through the neuraxis. -

Nucleus Dorsalis Superficialis (Lateralis Dorsalis) of the Thalamus and the Limbic System in Man
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.7.765 on 1 July 1974. Downloaded from Joutrnal of Neur)ology, Neurosurgery, and Psychiatry, 1974, 37, 765-789 Nucleus dorsalis superficialis (lateralis dorsalis) of the thalamus and the limbic system in man J. M. VAN BUREN AND R. C. BORKE Fr-om the Surgical Neurology Branch, National Institute of Neurological Diseases and Str-oke, National Institutes of Health, Bethesda, Maryland, U.S.A. SYNOPSIS Although the earlier supposition was that the n. dorsalis superficialis (n. lateralis dorsalis) of the thalamus projected to the parietal region, more recent evidence has linked it to the posterior cingulate gyrus and possibly adjacent regions near the splenium of the corpus callosum. An afferent supply from lower levels was in more doubt, although some report had been made of cell and fibre degeneration in the n. dorsalis superficialis after extensive temporal resections and section of the fornix in lower primates. The five human hemispheres of the present study all had lesions of long duration below the level of the splenium of the corpus callosum in the posteromedial temporal region. All showed marked degeneration in the fornix and n. dorsalis superficialis. In favourably Protected by copyright. stained cases, gliotic fascicles could be followed from the descending column of the fornix to the n. dorsalis superficialis via the region lateral to the stria medullaris thalami. The cell loss in the nucleus thus appeared to be an instance of anterograde transynaptic degeneration. These cases provided an interesting instance in which human infarctions provided natural lesions that would have been hard to duplicate in experimental animals. -

Neuroimaging Evidence Implicating Cerebellum in the Experience of Hypercapnia and Hunger for Air
Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air Lawrence M. Parsons*†, Gary Egan‡, Mario Liotti*, Stephen Brannan*, Derek Denton‡, Robert Shade§, Rachael Robillard¶, Lisa Madden§, Bart Abplanalp¶, and Peter T. Fox* *Research Imaging Center, University of Texas Health Science Center, San Antonio, TX 78284; ‡Howard Florey Institute of Experimental Physiology and Medicine, University of Melbourne, Parkville, Victoria 3052, Australia; §Southwest Foundation for Biomedical Research, P. O. Box 760549, San Antonio, TX 78245-0549; and ¶Departments of Psychology and Educational Psychology, University of Texas, Austin, TX 78712 Contributed by Derek Denton, December 20, 2000 Recent neuroimaging and neurological data implicate cerebellum areas (20–23). Anatomical tracer labeling data in rat (24) in nonmotor sensory, cognitive, vegetative, and affective func- indicate that a number of cerebellar areas send and͞or receive tions. The present study assessed cerebellar responses when the projections from the VRG, which contains the structures nec- urge to breathe is stimulated by inhaled CO2. Ventilation changes essary for respiratory rhythm generation (25–30). The cerebellar follow arterial blood partial pressure CO2 changes sensed by the areas connected to the VRG are quadrangular (VI), central medullary ventral respiratory group (VRG) and hypothalamus, (III), lingula (I, II), and inferior semilunar (Crus II) lobules, as entraining changes in midbrain, pons, thalamus, limbic, paralimbic, well as fastigial nucleus, interposed nucleus, and dentate nuclei, and insular regions. Nearly all these areas are known to connect the output nuclei for the vermal, intermediate, and lateral anatomically with the cerebellum. Using positron emission tomog- regions of cerebellum. Moreover, cerebellum has known con- raphy, we measured regional brain blood flow during acute CO2- nectivity to the hypothalamus, thalamus, pons, and midbrain induced breathlessness in humans. -

Control of Cerebellar Granule Cell Output by Sensory-Evoked Golgi Cell Inhibition
Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition Ian Duguid1,2,3, Tiago Branco1,4, Paul Chadderton5, Charlotte Arlt, Kate Powell6, and Michael Häusser3 Wolfson Institute for Biomedical Research and Department of Neuroscience, Physiology, and Pharmacology, University College London, London WC1E 6BT, United Kingdom Edited by Masao Ito, RIKEN Brain Science Institute, Wako, Japan, and approved September 1, 2015 (received for review May 25, 2015) Classical feed-forward inhibition involves an excitation–inhibition Results sequence that enhances the temporal precision of neuronal re- Sensory-Evoked Phasic and Spillover Golgi Cell Inhibition Precedes sponses by narrowing the window for synaptic integration. In Mossy Fiber Excitation in Granule Cells. Cerebellar granule cells the input layer of the cerebellum, feed-forward inhibition is thought receive direct phasic and indirect or “spillover” GABAergic in- to preserve the temporal fidelity of granule cell spikes during mossy put from Golgi cells (6, 16, 20, 21). To investigate the temporal fiber stimulation. Although this classical feed-forward inhibitory cir- dynamics of sensory-evoked inhibition in vivo, we recorded cuit has been demonstrated in vitro, the extent to which inhibition spontaneous and sensory-evoked excitatory (Vhold = −70 mV) shapes granule cell sensory responses in vivo remains unresolved. and inhibitory (Vhold = 0 mV) currents from the same granule Here we combined whole-cell patch-clamp recordings in vivo and cells in Crus II (Fig. 1 A–D). Granule cells were identified based dynamic clamp recordings in vitro to directly assess the impact of on their characteristic electrophysiological properties (Table S1), Golgi cell inhibition on sensory information transmission in the depth from the pial surface (>250 μm), and morphology (Fig. -
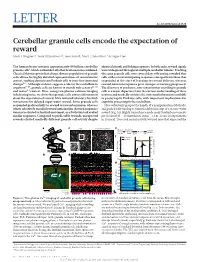
Cerebellar Granule Cells Encode the Expectation of Reward Mark J
LETTER doi:10.1038/nature21726 Cerebellar granule cells encode the expectation of reward Mark J. Wagner1*, Tony Hyun Kim1,2*, Joan Savall1, Mark J. Schnitzer1,3 & Liqun Luo1 The human brain contains approximately 60 billion cerebellar identical stimuli and licking responses. In both tasks, reward signals granule cells1, which outnumber all other brain neurons combined. were widespread throughout multiple cerebellar lobules. Tracking Classical theories posit that a large, diverse population of granule the same granule cells over several days of learning revealed that cells allows for highly detailed representations of sensorimotor cells with reward-anticipating responses emerged from those that context, enabling downstream Purkinje cells to sense fine contextual responded at the start of learning to reward delivery, whereas changes2–6. Although evidence suggests a role for the cerebellum in reward-omission responses grew stronger as learning progressed. cognition7–10, granule cells are known to encode only sensory11–13 The discovery of predictive, non-sensorimotor encoding in granule and motor14 context. Here, using two-photon calcium imaging cells is a major departure from the current understanding of these in behaving mice, we show that granule cells convey information neurons and markedly enriches the contextual information available about the expectation of reward. Mice initiated voluntary forelimb to postsynaptic Purkinje cells, with important implications for movements for delayed sugar-water reward. Some granule cells cognitive processing in the cerebellum. responded preferentially to reward or reward omission, whereas Mice voluntarily grasped the handle of a manipulandum (Methods) others selectively encoded reward anticipation. Reward responses and pushed it forward up to 8 mm for delayed receipt of a sucrose-water were not restricted to forelimb movement, as a Pavlovian task evoked reward (Fig. -

The Cerebellum: 3
41 The Cerebellum: 3. Anatomic-MR Correlation in the Coronal Plane Gary A. Press 1 Thin (5-mm) coronal high-field (1.5-T) MR images of four human brain specimens and James W. Murakami2 14 normal volunteers were correlated with myelin-stained microtomic sections of the Eric Courchesne2 specimen cerebella. The primary white-matter tracts innervating several hemispheric Marjorie Grafe3 (posterior quadrangular, superior, and inferior semilunar, gracile, biventer, tonsil) and John R. Hesselink1 vermian (declive, folium, tuber) lobules are oriented perpendicularly to the coronal plane of section and are shown well on proton-density-weighted (long TR/short TE) and T2- weighted (long TR/Iong TE) spin-echo images, which provide excellent contrast between gray and white matter. Several of the surface sulci and fissures of the cerebellar hemispheres (including the superior posterior, horizontal, secondary, and posterolateral fissures) also course perpendicular to the coronal plane and are depicted well on T1- weighted (short TR/short TE) and T2-weighted images, which maximize contrast be tween CSF and parenchyma. The opportunity for side-to-side comparison of the hemi spheres is a distinct advantage of the coronal view. Nevertheless, more obliquely oriented surfaces (preculminate, primary, inferior posterior, inferior anterior, and intra biventral fissures) and deep hemispheric structures (primary white-matter tracts to central, anterior quadrangular, and floccular lobules) may be obscured by volume averaging in the coronal plane; moreover, much of the finer anatomy of the vermis is depicted poorly. The constant surface and deep anatomy of the cerebellum revealed on coronal images in normal volunteers encourages detailed mapping. MR imaging in the coronal plane should be especially useful in identifying, localizing, and quantifying normal and abnormal morphologic differences between the cerebellar hemispheres. -

Anatomy of the Cerebellum Computational Models of Neural
Anatomy of the Cerebellum Computational Models of neural Systems Lecture 2.1 David S. Touretzky September, 2015 First Look cerebellum 09/09/15 Computational Models of Neural Systems 2 Lateral View 09/09/15 Computational Models of Neural Systems 3 Ventral View 09/09/15 Computational Models of Neural Systems 4 Basic Facts About the Cerebellum ● Latin for ªlittle brainº. ● An older brain area, with a simple, regular architecture. ● Makes up 10% of brain volume, but contains over 50% of the brain©s neurons and 4X the neurons of the cerebral cortex. ● Huge fan-in: 40X as many axons enter the cerebellum as exit from it. ● Necessary for smooth, accurate performance of motor actions. ● Example: moving your arm rapidly in a circle. – Involves many muscles in the arm, trunk, and legs. ● People can still move without a cerebellum, but their actions will not be coordinated. There can be overshoots and oscillations. 09/09/15 Computational Models of Neural Systems 5 Cortical Projections to Cerebellum From Strick et al., Annual review of Neuroscience (2009), adapted from Glickstein et al. (1985) J. Comparative Neurology 09/09/15 Computational Models of Neural Systems 6 Three Cerebellar Lobes ● Anterior (divided into 3 lobules) ● Posterior (divided into 6 lobules) ● Flocculonodular 09/09/15 Computational Models of Neural Systems 7 10 Lobules Lingula, Central, Culmen, Declive, Folium, Tuber, Pyramis, Uvula, Tonsil, Flocculonodular 09/09/15 Computational Models of Neural Systems 8 8 of the 10 Lobules 1. Lingula 2. Central Lobule 3. Culmen 4. Declive 5. -

Is Composed from Spinal Cord and Brain
doc. MUDr. Adriana Boleková, PhD. MVDr. Natália Hvizdošová, PhD. CENTRAL NERVOUS SYSTEM – is composed from spinal cord and brain SPINAL CORD cranial border: foramen magnum, pyramidal decussation, exit of first pair of spinal nerves caudal border: level of L1 – L2 vertebrae medullary cone – filum terminale (S2) – cauda equina enlargements: cervical enlargement (C5 – Th1): origin of nerves for upper extremity – brachial plexus lumbosacral enlargement (L1 – S2): origin of nerves for lower extremity – lumbosacral plexus external features: anterior median fissure anterolateral sulcus – anterior roots of spinal nn. posterolateral sulcus – posterior roots of spinal nn. posterior median sulcus posterior intermediate sulcus internal features: White matter anterior funiculus (between anterior median fissure and anterolateral sulcus) lateral funiculus (between anterolateral and posterolateral sulci) posterior funiculus (between posterolateral sulcus and posterior median sulcus) fasciculus gracilis fasciculus cuneatus Gray matter anterior (ventral) horn – motor function: Rexed laminae I – VI lateral horn – serves to visceral function: Rexed lamina VII dorsal (posterior) horn – sensory information: Rexed laminae VIII – IX central grey matter – interneurons: around central canal Rexed lamina X Central canal cranially opens into IV. ventricle caudally expands into terminal ventricle vessels of spinal cord: Arteries: spinal brr. from surrounding arteries – anterior radicular aa., posterior radicular aa.: posterior spinal aa. (in posterolateral -

Neuroimaging Evidence Implicating Cerebellum in Support of Sensory Cognitive Processes Associated with Thirst
Neuroimaging evidence implicating cerebellum in support of sensory͞cognitive processes associated with thirst Lawrence M. Parsons*†, Derek Denton‡, Gary Egan‡, Michael McKinley‡, Robert Shade‡, Jack Lancaster*, and Peter T. Fox* *Research Imaging Center, Medical School, University of Texas Health Science Center at San Antonio, Floyd Curl Drive, San Antonio, TX 78284; ‡Howard Florey Institute of Experimental Physiology and Medicine, University of Melbourne, Parkville, Victoria 3052, Australia; and §Southwest Foundation for Biomedical Research, P.O. Box 760549, San Antonio, TX 78245-0549 Contributed by Derek A. Denton, December 13, 1999 Recent studies implicate the cerebellum, long considered strictly a and tactile discrimination, kinesthetic sensation, and working motor control structure, in cognitive, sensory, and affective phe- memory, among other processes (6–12). It has been proposed nomenon. The cerebellum, a phylogenetically ancient structure, that the lateral cerebellum may be activated during several has reciprocal ancient connections to the hypothalamus, a struc- motor, perceptual, and cognitive processes specifically because ture important in vegetative functions. The present study investi- of the requirement to monitor and adjust the acquisition of gated whether the cerebellum was involved in vegetative func- sensory data (2, 13). Furthermore, there are reports suggesting tions and the primal emotions engendered by them. Using positron the involvement of posterior vermal cerebellum in affect (14, 15). emission tomography, we examined the effects on the cerebellum The findings implicating the cerebellum in sensory processing of the rise of plasma sodium concentration and the emergence of and emotional states make it of great interest to examine thirst in 10 healthy adults. The correlation of regional cerebral whether the cerebellum has a role in basic vegetative functions blood flow with subjects’ ratings of thirst showed major activation and the primal emotions thus generated, particularly given the in the vermal central lobule. -

Plexin-B2 Controls the Development of Cerebellar Granule Cells
The Journal of Neuroscience, April 4, 2007 • 27(14):3921–3932 • 3921 Development/Plasticity/Repair Plexin-B2 Controls the Development of Cerebellar Granule Cells Roland H. Friedel,1*Ge´raldine Kerjan,2* Helen Rayburn,1 Ulrich Schu¨ller,3 Constantino Sotelo,2,4 Marc Tessier-Lavigne,1 and Alain Che´dotal2 1Department of Biological Sciences, Howard Hughes Medical Institute, Stanford University, Stanford, California 94305, 2Centre National de la Recherche Scientifique, Unite´ Mixte de Recherche 7102, Universite´ Paris 6, 75005 Paris, France, 3Department of Pediatric Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts 02115, and 4Ca´tedra de Neurobiologı´a del Desarrollo “Remedios Caro Almela,” Instituto de Neurociencias de Alicante, Universidad Miguel Herna´ndez de Elche, Consejo Superior de Investigaciones Cientı´ficas, 03550 San Juan de Alicante, Alicante, Spain Cerebellar granule cell progenitors proliferate postnatally in the upper part of the external granule cell layer (EGL) of the cerebellum. Postmitotic granule cells differentiate and migrate, tangentially in the EGL and then radially through the molecular and Purkinje cell layers. The molecular control of the transition between proliferation and differentiation in cerebellar granule cells is poorly understood. We show here that the transmembrane receptor Plexin-B2 is expressed by proliferating granule cell progenitors. To study Plexin-B2 function, we generated a targeted mutation of mouse Plexin-B2. Most Plexin-B2Ϫ/Ϫ mutants die at birth as a result of neural tube closure defects. Some mutants survive but their cerebellum cytoarchitecture is profoundly altered. This is correlated with a disorganization of the timing of granule cell proliferation and differentiation in the EGL. Many differentiated granule cells migrate inside the cerebellum and keep proliferating. -

On the Function of the Floccular Complex of the Vertebrate Cerebellum: Implications in Paleoneuroanatomy
On the function of the floccular complex of the vertebrate cerebellum: implications in paleoneuroanatomy Sérgio Filipe Ferreira Cardoso Dissertação para obtenção do Grau de Mestre em Paleontologia Orientador: Doutor Rui Alexandre Ferreira Castanhinha Co-orientadores: Doutor Ricardo Miguel Nóbrega Araújo Prof. Doutor Miguel Telles Antunes On the function of the floccular complex of the vertebrate cerebellum: implications in paleoneuroanatomy Sérgio Filipe Ferreira Cardoso Dissertação para obtenção do Grau de Mestre em Paleontologia Orientador: Doutor Rui Alexandre Ferreira Castanhinha Co-orientadores: Doutor Ricardo Miguel Nóbrega Araújo Prof. Doutor Miguel Telles Antunes Successfully defended on 18th November 2015 at FCT-UNL Campus, Portugal, before a juri presided over by: Doutor Paulo Alexandre Rodrigues Roque Legoinha and consisting of: Doutor Gabriel José Gonçalves Martins Doutor Rui Alexandre Ferreira Castanhinha I II Direitos de autor - Copyright Os direitos de autor deste documento pertencem a Sérgio Filipe Ferreira Cardoso, à FCT/UNL, à UNL e à UÉ. A Faculdade de Ciências e Tecnologia, a Universidade Nova de Lisboa e a Universidade de Évora têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. Two peer-reviewed abstracts, resulting from this study, were accepted for oral communications (Appendix II). Ferreira-Cardoso, S., Araújo, R., Castanhinha, R., Walsh, S., Martins, R.M.S., Martins, G.G.