Lorcifera, an Under Known Phyla: First Record of Higgins Larva of Armorloricus (Loricifera: Nanaloricidae) from Indian Waters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Platyhelminthes, Nemertea, and "Aschelminthes" - A
BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. III - Platyhelminthes, Nemertea, and "Aschelminthes" - A. Schmidt-Rhaesa PLATYHELMINTHES, NEMERTEA, AND “ASCHELMINTHES” A. Schmidt-Rhaesa University of Bielefeld, Germany Keywords: Platyhelminthes, Nemertea, Gnathifera, Gnathostomulida, Micrognathozoa, Rotifera, Acanthocephala, Cycliophora, Nemathelminthes, Gastrotricha, Nematoda, Nematomorpha, Priapulida, Kinorhyncha, Loricifera Contents 1. Introduction 2. General Morphology 3. Platyhelminthes, the Flatworms 4. Nemertea (Nemertini), the Ribbon Worms 5. “Aschelminthes” 5.1. Gnathifera 5.1.1. Gnathostomulida 5.1.2. Micrognathozoa (Limnognathia maerski) 5.1.3. Rotifera 5.1.4. Acanthocephala 5.1.5. Cycliophora (Symbion pandora) 5.2. Nemathelminthes 5.2.1. Gastrotricha 5.2.2. Nematoda, the Roundworms 5.2.3. Nematomorpha, the Horsehair Worms 5.2.4. Priapulida 5.2.5. Kinorhyncha 5.2.6. Loricifera Acknowledgements Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS This chapter provides information on several basal bilaterian groups: flatworms, nemerteans, Gnathifera,SAMPLE and Nemathelminthes. CHAPTERS These include species-rich taxa such as Nematoda and Platyhelminthes, and as taxa with few or even only one species, such as Micrognathozoa (Limnognathia maerski) and Cycliophora (Symbion pandora). All Acanthocephala and subgroups of Platyhelminthes and Nematoda, are parasites that often exhibit complex life cycles. Most of the taxa described are marine, but some have also invaded freshwater or the terrestrial environment. “Aschelminthes” are not a natural group, instead, two taxa have been recognized that were earlier summarized under this name. Gnathifera include taxa with a conspicuous jaw apparatus such as Gnathostomulida, Micrognathozoa, and Rotifera. Although they do not possess a jaw apparatus, Acanthocephala also belong to Gnathifera due to their epidermal structure. ©Encyclopedia of Life Support Systems (EOLSS) BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. -

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -
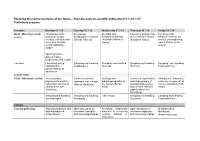
Exploring the Marine Meiofauna of the Azores – from Discovery to Scientific Publication (15.7.-24.7.19) Preliminary Program
Exploring the marine meiofauna of the Azores – from discovery to scientific publication (15.7.-24.7.19) Preliminary program: Schedule Monday 15.7.19 Tuesday 16.7.19 Wednesday 17.7.19 Thursday 18.7.19 Friday 19.7.19 09.00 - Morning session Introduction to the Meiofaunal Intertidal and Taxonomy and diversity The Proseriata Lecture workshop (people, Scalidophora (Andreas meiofaunal annelids of meiofaunal molluscs (Platyhelminthes): the facilities, schedule and Schmidt-Rhaesa) (Alejandro Martínez (Katharina Jörger) interest of unappealing aims; Ana Ricardo Garcia) worms (Marco Curini- Costa/ Katharina Galetti) Jörger) Opening lecture: Azores marine biodiversity (Ana Costa) Lab work Laboratory set-up, Sampling and handling Sampling and handling Sampling and handling Sampling and handling handling and Scalidophora Annelida Mollusca Platyhelminthes documentation of specimens LUNCH TIME 14.00 - Afternoon session Acoelomorpha – Marine nematodes: Geology and Taxonomy, systematics Rhabdocoel flatworms, phylogenetic position, taxonomy and ecology paleobiogeography of and biogeography of a diverse component of systematic and how to (Alberto Navarrete) the Azores (Sérgio meiofaunal Nemertea marine meiofauna (Tom identify them (Ulf Avila) and relevant research Artois) Jondelius) opportunities (Jon Norenburg) Sampling and handling Sampling and handling Lab session Sampling and handling Sampling and handling Acoelomorpha Nematoda Nemertea Platyhelminthes DINNER Evening gathering Sampling strategies and Open discussion on Adressing biodiversity Open discussion -

The First Metazoa Living in Permanently Anoxic Conditions
Danovaroet al. BMC Biology 2010,8:30 http://www.biomedcentral.eom/1741-7007/8/30 BMC Biology RESEARCH ARTICLE Open Access The first metazoa living in permanently anoxic conditions Roberto Danovaro*1, Antonio Dell'Anno1, Antonio Pusceddu1, Cristina G am bi1, Iben Heiner2 and Reinhardt Mobjerg Kristensen 2 A bstract Background:Several unicellular organisms (prokaryotes and protozoa) can live under permanently anoxic conditions. Although a few metazoans can survive temporarily in the absence of oxygen, it is believed that multi-cellular organisms cannot spend their entire life cycle without free oxygen. Deep seas include some of the most extreme ecosystems on Earth, such as the deep hypersaline anoxic basins of the Mediterranean Sea. These are permanently anoxic systems inhabited by a huge and partly unexplored microbial biodiversity. R esults:During the last ten years three oceanographic expeditions were conducted to search for the presence of living fauna in the sediments of the deep anoxic hypersaline L'Atalante basin (Mediterranean Sea). We report here that the sediments of the L'Atalante basin are inhabited by three species of the animal phylum Loricifera(Spinoloricus nov. sp., Rugiloricus nov. sp. andPliciloricus nov. sp.) new to science. Using radioactive tracers, biochemical analyses, guantitative X-ray microanalysis and infrared spectroscopy, scanning and transmission electron microscopy observations on ultra-sections, we provide evidence that these organisms are metabolically active and show specific adaptations to the extreme conditions of the deep basin, such as the lack of mitochondria, and a large number of hydrogenosome-like organelles, associated with endosymbiotic prokaryotes. Conclusions:This is the first evidence of a metazoan life cycle that is spent entirely in permanently anoxic sediments. -

Loricifera from the Deep Sea at the Galápagos Spreading Center, with a Description of Spinoloricus Turbatio Gen. Et Sp. Nov. (Nanaloricidae)
Helgol Mar Res (2007) 61:167–182 DOI 10.1007/s10152-007-0064-9 ORIGINAL ARTICLE Loricifera from the deep sea at the Galápagos Spreading Center, with a description of Spinoloricus turbatio gen. et sp. nov. (Nanaloricidae) Iben Heiner · Birger Neuhaus Received: 1 August 2006 / Revised: 26 January 2007 / Accepted: 29 January 2007 / Published online: 10 March 2007 © Springer-Verlag and AWI 2007 Abstract Specimens of a new species of Loricifera, nov. are characterized by six rectangular plates in the Spinoloricus turbatio gen. et sp. nov., have been col- seventh row with two teeth, an indistinct honeycomb lected at the Galápagos Spreading Center (GSC) dur- sculpture and long toes with little mucrones. The SO ing the cruise SO 158, which is a part of the 158 cruise has yielded a minimum of ten new species of MEGAPRINT project. The new genus is positioned in Loricifera out of only 42 specimens. These new species the family Nanaloricidae together with the three belong to two diVerent orders, where one being new to already described genera Nanaloricus, Armorloricus science, and three diVerent families. This result indi- and Phoeniciloricus. The postlarvae and adults of Spi- cates a high diversity of loriciferans at the GSC. Nearly noloricus turbatio gen. et sp. nov. are characterized by all the collected loriciferans are in a moulting stage, a mouth cone with eight oral ridges and basally with a hence there is a new stage inside the present stage. This cuticular reinforcement named mouth cone pleat; prolongation of life stages and the occurrence of multi- eighth row with 30 whip-like spinoscalids and 30 “alter- ple life stages inside each other are typical of deep-sea nating” plates; thorax with eight single and seven dou- loriciferans. -

The Anatomy, Affinity, and Phylogenetic Significance of Markuelia
EVOLUTION & DEVELOPMENT 7:5, 468–482 (2005) The anatomy, affinity, and phylogenetic significance of Markuelia Xi-ping Dong,a,Ã Philip C. J. Donoghue,b,Ã John A. Cunningham,b,1 Jian-bo Liu,a andHongChengc aDepartment of Earth and Space Sciences, Peking University, Beijing 100871, China bDepartment of Earth Sciences, University of Bristol, Wills Memorial Building, Queen’s Road, Bristol BS8 1RJ, UK cCollege of Life Sciences, Peking University, Beijing 100871, China ÃAuthors for correspondence (email: [email protected], [email protected]) 1Present address: Department of Earth and Ocean Sciences, University of Liverpool, 4 Brownlow Street, Liverpool L69 3GP, UK. SUMMARY The fossil record provides a paucity of data on analyses have hitherto suggested assignment to stem- the development of extinct organisms, particularly for their Scalidophora (phyla Kinorhyncha, Loricifera, Priapulida). We embryology. The recovery of fossilized embryos heralds new test this assumption with additional data and through the insight into the evolution of development but advances are inclusion of additional taxa. The available evidence supports limited by an almost complete absence of phylogenetic stem-Scalidophora affinity, leading to the conclusion that sca- constraint. Markuelia is an exception to this, known from lidophorans, cyclonerualians, and ecdysozoans are primitive cleavage and pre-hatchling stages as a vermiform and direct developers, and the likelihood that scalidophorans are profusely annulated direct-developing bilaterian with terminal primitively metameric. circumoral and posterior radial arrays of spines. Phylogenetic INTRODUCTION et al. 2004b). Very early cleavage-stage embryos of presumed metazoans and, possibly, bilaterian metazoans, have been re- The fossil record is largely a record of adult life and, thus, covered from the late Neoproterozoic (Xiao et al. -

Animal Phylogeny and the Ancestry of Bilaterians: Inferences from Morphology and 18S Rdna Gene Sequences
EVOLUTION & DEVELOPMENT 3:3, 170–205 (2001) Animal phylogeny and the ancestry of bilaterians: inferences from morphology and 18S rDNA gene sequences Kevin J. Peterson and Douglas J. Eernisse* Department of Biological Sciences, Dartmouth College, Hanover NH 03755, USA; and *Department of Biological Science, California State University, Fullerton CA 92834-6850, USA *Author for correspondence (email: [email protected]) SUMMARY Insight into the origin and early evolution of the and protostomes, with ctenophores the bilaterian sister- animal phyla requires an understanding of how animal group, whereas 18S rDNA suggests that the root is within the groups are related to one another. Thus, we set out to explore Lophotrochozoa with acoel flatworms and gnathostomulids animal phylogeny by analyzing with maximum parsimony 138 as basal bilaterians, and with cnidarians the bilaterian sister- morphological characters from 40 metazoan groups, and 304 group. We suggest that this basal position of acoels and gna- 18S rDNA sequences, both separately and together. Both thostomulids is artifactal because for 1000 replicate phyloge- types of data agree that arthropods are not closely related to netic analyses with one random sequence as outgroup, the annelids: the former group with nematodes and other molting majority root with an acoel flatworm or gnathostomulid as the animals (Ecdysozoa), and the latter group with molluscs and basal ingroup lineage. When these problematic taxa are elim- other taxa with spiral cleavage. Furthermore, neither brachi- inated from the matrix, the combined analysis suggests that opods nor chaetognaths group with deuterostomes; brachiopods the root lies between the deuterostomes and protostomes, are allied with the molluscs and annelids (Lophotrochozoa), and Ctenophora is the bilaterian sister-group. -

Abstracts of the 5Th Annual Meeting of the Gesellschaft Für Biologische Systematik (Society for Biological Systematics)
Org. Divers. Evol. 3, Electr. Suppl. 2: 1 - 31 (2003) © Gesellschaft für Biologische Systematik http://senckenberg.de/odes/03-02.htm Abstracts of the 5th Annual Meeting of the Gesellschaft für Biologische Systematik (Society for Biological Systematics) Roland Melzer & Michael Schrödl (eds) Electr. Suppl. 2. - to: Org. Divers. Evol. 3(1): 72. 2003 Preface In the following, 36 short communications are given that were presented as lectures or posters at the 5th Annual Meeting of the GfBS (see www.gfbs-home.de/) held in Munich, 18-20 September 2002. The meeting was organized by the Botanische Staatssammlung München (BSM), Zoologi- sche Staatssammlung München (ZSM), Department Biologie I of Ludwig-Maximilians-Universität München (LMU), and the GeoBio-CenterLMU. The main topics were (i) theory of systematics, mechanisms of evolution, molecular systematics; (ii) biodiversity and collection information systems; and (iii) special issues concerning various taxa, in particular Coniferales, Anthozoa, Brachiopoda, Mollusca, Acanthocephala, Nematoda, Arthropo- da and Vertebrata. An invited lecture was given by Bernd Schierwater ("Molecular development and molecule morphology in systematics: back to the future?"), and a public lecture by Ragnar Kinzelbach ("Der Seidenschwanz Bombycilla garrulus in Europa vor 1758"). In addition, a curators' meeting – organized by Marion Kotrba (ZSM) – and a meeting of the "Young Systematists" – organized by Sybille Seifried (Oldenburg) – were held in parallel sessions. The organizers thank all those persons who spent -

Ecdysozoan Phylogeny and Bayesian Inference: First Use of Nearly Complete 28S and 18S Rrna Gene Sequences to Classify the Arthro
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 31 (2004) 178–191 www.elsevier.com/locate/ympev Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kinq Jon M. Mallatt,a,* James R. Garey,b and Jeffrey W. Shultzc a School of Biological Sciences, Washington State University, Pullman, WA 99164-4236, USA b Department of Biology, University of South Florida, 4202 East Fowler Ave. SCA110, Tampa, FL 33620, USA c Department of Entomology, University of Maryland, College Park, MD 20742, USA Received 4 March 2003; revised 18 July 2003 Abstract Relationships among the ecdysozoans, or molting animals, have been difficult to resolve. Here, we use nearly complete 28S + 18S ribosomal RNA gene sequences to estimate the relations of 35 ecdysozoan taxa, including newly obtained 28S sequences from 25 of these. The tree-building algorithms were likelihood-based Bayesian inference and minimum-evolution analysis of LogDet-trans- formed distances, and hypotheses were tested wth parametric bootstrapping. Better taxonomic resolution and recovery of estab- lished taxa were obtained here, especially with Bayesian inference, than in previous parsimony-based studies that used 18S rRNA sequences (or 18S plus small parts of 28S). In our gene trees, priapulan worms represent the basal ecdysozoans, followed by ne- matomorphs, or nematomorphs plus nematodes, followed by Panarthropoda. Panarthropoda was monophyletic with high support, although the relationships among its three phyla (arthropods, onychophorans, tardigrades) remain uncertain. The four groups of arthropods—hexapods (insects and related forms), crustaceans, chelicerates (spiders, scorpions, horseshoe crabs), and myriapods (centipedes, millipedes, and relatives)—formed two well-supported clades: Hexapoda in a paraphyletic crustacea (Pancrustacea), and ÔChelicerata + MyriapodaÕ (a clade that we name ÔParadoxopodaÕ). -

The Early History of the Metazoa—A Paleontologist's Viewpoint
ISSN 20790864, Biology Bulletin Reviews, 2015, Vol. 5, No. 5, pp. 415–461. © Pleiades Publishing, Ltd., 2015. Original Russian Text © A.Yu. Zhuravlev, 2014, published in Zhurnal Obshchei Biologii, 2014, Vol. 75, No. 6, pp. 411–465. The Early History of the Metazoa—a Paleontologist’s Viewpoint A. Yu. Zhuravlev Geological Institute, Russian Academy of Sciences, per. Pyzhevsky 7, Moscow, 7119017 Russia email: [email protected] Received January 21, 2014 Abstract—Successful molecular biology, which led to the revision of fundamental views on the relationships and evolutionary pathways of major groups (“phyla”) of multicellular animals, has been much more appre ciated by paleontologists than by zoologists. This is not surprising, because it is the fossil record that provides evidence for the hypotheses of molecular biology. The fossil record suggests that the different “phyla” now united in the Ecdysozoa, which comprises arthropods, onychophorans, tardigrades, priapulids, and nemato morphs, include a number of transitional forms that became extinct in the early Palaeozoic. The morphology of these organisms agrees entirely with that of the hypothetical ancestral forms reconstructed based on onto genetic studies. No intermediates, even tentative ones, between arthropods and annelids are found in the fos sil record. The study of the earliest Deuterostomia, the only branch of the Bilateria agreed on by all biological disciplines, gives insight into their early evolutionary history, suggesting the existence of motile bilaterally symmetrical forms at the dawn of chordates, hemichordates, and echinoderms. Interpretation of the early history of the Lophotrochozoa is even more difficult because, in contrast to other bilaterians, their oldest fos sils are preserved only as mineralized skeletons.